Specialisation impacts the European Contract Research Services market
Posted: 7 April 2008 | Dr. Amarpreet S Dhiman, Drug Discovery Technologies, Healthcare (EMEA) and Industry Analyst, Drug Discovery and Clinical Diagnostics Frost & Sullivan and Programme Leader | No comments yet
Research within the pharmaceutical industry has tripled in the past 25 years, with the pipelines of the top companies doubling. Stricter regulations, guidelines, price and reimbursement legislation all result in a changing business environment. The growing market in drug development and increase in research and development (R&D) investment, including that of small service companies; coupled with an increase in development costs; the importance of timely development of new products and the aim of reducing time-to-market are all important financial considerations for achieving business growth.
Research within the pharmaceutical industry has tripled in the past 25 years, with the pipelines of the top companies doubling. Stricter regulations, guidelines, price and reimbursement legislation all result in a changing business environment. The growing market in drug development and increase in research and development (R&D) investment, including that of small service companies; coupled with an increase in development costs; the importance of timely development of new products and the aim of reducing time-to-market are all important financial considerations for achieving business growth.
Research within the pharmaceutical industry has tripled in the past 25 years, with the pipelines of the top companies doubling. Stricter regulations, guidelines, price and reimbursement legislation all result in a changing business environment. The growing market in drug development and increase in research and development (R&D) investment, including that of small service companies; coupled with an increase in development costs; the importance of timely development of new products and the aim of reducing time-to-market are all important financial considerations for achieving business growth.
While pharmaceutical and biotech R&D spending is surpassing $36 billion today and blockbuster drug development is levelling off, Contract Research Organisations (CROs) are moving in the direction of becoming either niche specific or large service providers. In the 1980s, the pharmaceutical industry came under a great deal of scrutiny from the FDA, which was closely monitoring the way clinical trials were conducted by pharmaceutical manufacturers. The regulatory atmosphere at that time was putting pressure on the pharmaceutical industry to outsource its processes. Many ‘new’ outsourcers relocated from the pharmaceutical companies and specialised in one particular area, staying regional and focussing on clinical monitoring, data management or project coordination. The last part of the 20th Century witnessed aggressive mergers and acquisitions among pharmaceutical and biotechnology companies as well many other bodies. As many CROs merged and grew larger, they had become full-service global trial managers. The emergence of truly global service companies with strong track records has tremendously matured the outsourcing process. The CRO industry is advancing through global expansion, process improvement and innovative technology.
Although cost efficiency is a significant driver, outsourcing has become increasingly focussed on emphasising the other attributes of importance to CROs. Companies wanting to generate new ideas and be on the verge of breakthrough technologies have traditionally been willing to forge alliances with outside organisations, creating collaborative long-term relationships, which historically may have had roots in academia or biotech firms forming pseudo-strategic alliances. Consequently, these provide opportunities to concentrate on core competencies, whereby outsourcing of non-core and transactional tasks can allow greater focus on specific areas that are less commodity-driven. Expertise and capabilities, rather than attempting to compete by being a low-cost provider, are other characteristics, as most companies recognise that saving a small amount of money on fees could ultimately cost the firm more in project delays. This strategy has become a successful trend for many large CROs, allowing them to compete primarily on the basis of price as they increasingly emphasise their unique capabilities and how those can help them complete projects on or before schedule.
The CRO industry today faces many large full-service organisations providing global capabilities. A large number of pharmaceutical companies are becoming much more selective in their choice of CROs. Whereas the largest CROs are often an automatic choice for major projects, pharmaceutical companies have increasingly (in recent decades) turned to smaller-sized organisations. These include niche participants, academic medical centres (AMCs) and teaching hospitals and laboratory service companies, which have the ability to be more responsive and flexible. These organisations have good geographic coverage, broad therapeutic coverage and years of experience in managing complex trials. They can also advise companies on realistic timelines for the clinical development of drugs in a range of therapeutic areas and on changes in regulatory guidelines. For this reason, they are ideally placed to handle major projects and yet retain a more personalised service.
Industry hurdles
Along with an increase in growth, the CRO industry is faced with new challenges. Several themes that have emerged suggest that the challenges CROs will face would relate to quality, efficiency; the need for therapeutic expertise; the increase in the number of trials; difficulty in recruitment; the globalisation of drug development; limitations on drug price increases; fewer blockbuster drugs; competition both within and outside the industry; sponsors tightening budgets; the need for better sponsor-CRO communication; the globalisation of trials and EU regulations. The challenge to drug sponsors and CROs is to collaboratively embrace the reality of outsourcing, but in the context of creating value and new core competencies of relationship and outsourcing management efficiency. With the growing demand to provide experienced research staff for every project in a business that is mostly a repeat business, this can often become difficult and lead to lengthened project completion times, lower data quality and, ultimately, client dissatisfaction. At the same time, sponsors are under greater pressure than ever before to fill their pipelines with new product candidates and quickly rush new drugs to market. Thus, while the client expectations are rising, the ability of CROs to fulfil them is declining. If relationships can begin with clear definitions of roles and responsibilities, there is a greater chance of success for both parties. The outcome of working together in a smart manner will lead to a reduction in cycle times and create repeat business for many clinical trial tasks. Given these considerations, companies in the CRO industry are seeing a transformed growth rate in revenue together with a positive future that is set to provide limitless opportunities for expansion.
Conclusions
In Europe, the nature of drug discovery is changing at a rapid rate. Genomics, proteomics, combinatorial chemistry and high throughput screening has produced a plethora of drug targets and provided a tremendous increase in the amount of data available to move from the phase of discovery towards development. That, in turn, requires that as CROs become more long-term partners for the pharmaceutical, biotechnology and the drug discovery industry, outsourcing strategies should be based on more strategic, operational considerations instead of solely financial reasons. For the market to experience continued growth for the future, aligned objectives need to be established between pharmaceutical and biotechnology companies and the CROs to achieve mutually common goals.
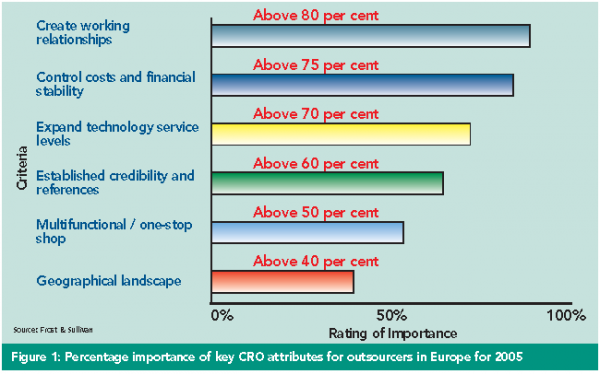

Background
Frost & Sullivan, a global growth consulting company, has been partnering with clients to support the development of innovative strategies for more than 40 years. The company’s industry expertise integrates growth consulting, growth partnership services, and corporate management training to identify and develop opportunities. Frost & Sullivan serves an extensive clientele that includes Global 1000 companies, emerging companies, and the investment community by providing comprehensive industry coverage that reflects a unique global perspective and combines ongoing analysis of markets, technologies, econometrics, and demographics.
Dr. Amarpreet S Dhiman
Drug Discovery Technologies, Healthcare (EMEA) and Industry Analyst, Drug Discovery and Clinical Diagnostics Frost & Sullivan and Programme Leader
Amarpreet joined Frost & Sullivan as research analyst in 2004. Working with top multinational companies and communicating with top level healthcare company executives in the drug discovery and clinical diagnostics field, Amarpreet is directly involved in project delivery, from launch to quality control phases according to a stringent production timeline.
In addition to writing a number of commercially penetrating articles and participating in several international seminars and conferences, he has finished profiling fast-growing emerging markets in the drug discovery and clinical diagnostics field, focusing on the whole value chain of the drug discovery and development process.
Amarpreet holds a Ph.D. in Dental Prosthetics from Queen Mary University (London) in addition to a Master’s Degree in Biomedical Engineering from Imperial College (London), and has published many papers in peer-reviewed scientific journals.









