Designing a program for early CNS development
Posted: 28 November 2006 | | No comments yet
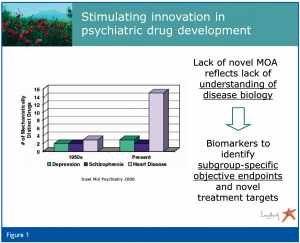
Only a few innovations have been made in recent decades with regard to psychiatric, and particularly antidepressant, drugs (Insel et al., 2006) (Figure 1). This conundrum reflects, at least partly, the lack of understanding of the disease biology. This poses a challenge not only to inventive drug development, but also to clinical practice, which faces remission rates of 30 per cent and less in patients given state-of-the-art pharmacological treatment for major depressive disorders (Trivedi et al., 2006). The situation is further aggravated by the exclusive current use of clinically based diagnostic criteria for major depression, which some critics view as ‘a pseudo-category, effectively homogenizing multiple expressions of depression’ (Parker, 2004).
Only a few innovations have been made in recent decades with regard to psychiatric, and particularly antidepressant, drugs (Insel et al., 2006) (Figure 1). This conundrum reflects, at least partly, the lack of understanding of the disease biology. This poses a challenge not only to inventive drug development, but also to clinical practice, which faces remission rates of 30 per cent and less in patients given state-of-the-art pharmacological treatment for major depressive disorders (Trivedi et al., 2006). The situation is further aggravated by the exclusive current use of clinically based diagnostic criteria for major depression, which some critics view as ‘a pseudo-category, effectively homogenizing multiple expressions of depression’ (Parker, 2004).
Only a few innovations have been made in recent decades with regard to psychiatric, and particularly antidepressant, drugs (Insel et al., 2006) (Figure 1). This conundrum reflects, at least partly, the lack of understanding of the disease biology. This poses a challenge not only to inventive drug development, but also to clinical practice, which faces remission rates of 30 per cent and less in patients given state-of-the-art pharmacological treatment for major depressive disorders (Trivedi et al., 2006). The situation is further aggravated by the exclusive current use of clinically based diagnostic criteria for major depression, which some critics view as ‘a pseudo-category, effectively homogenizing multiple expressions of depression’ (Parker, 2004).
In this situation, it may not come as a surprise that biological markers (biomarkers) associated with depression in one scientific article cannot be reproduced in subsequent studies. Ultimately, the core problem when searching for biomarkers is the unsatisfactory clinical description of patients, which is a prerequisite to making a robust and reproducible association between biological markers and the clinical phenotype.
It is anticipated that a revision of the current diagnostic classification for psychiatric disorders (the fifth Diagnostic and Statistical Manual, DSM-V) will consist of a more detailed clinical description of patients, including the course of the disease; the context in which it occurs; the familial background and comorbid disorders. Also, there is hope that the new classification will consider neurobiological features. However, availability of a revised diagnostic manual is not expected before 2011 – quite possibly even later.
The decision was therefore made to begin implementing a systematic biomarker discovery program that aims to achieve the following:
- Improve the link between clinical and preclinical data
- Help determine the optimal dose
- Obtain the best target populations for human Proof-of-Concept (PoC) and subsequent studies
From the onset a clarification of definitions is essential: The NIH working group has defined a biomarker as a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes or pharmacologic responses to a therapeutic intervention (Biomarkers Definitions Working Group, 2001). Functional categories include exposure, safety and efficacy biomarkers. If an efficacy marker can substitute for a clinical endpoint, it can serve as a surrogate endpoint in clinical trials.
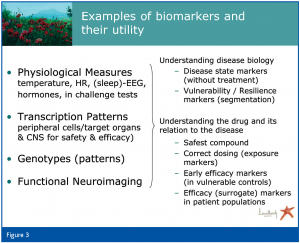
In our program the biomarker search is based primarily, but not exclusively, on assessment of physiological measures (under both baseline conditions and in challenge paradigms), transcription patterns (from target organs (CNS or other for safety issues) and peripheral cells), genotypes and neuroimaging, including both target occupancy and functional read-outs.
To strengthen the link between preclinical and clinical data, with regard to both safety and efficacy aspects, animal models to assess novel drug candidates should share at least some core biological features of the human disorder. This may be particularly relevant when testing compounds with a novel mechanism-of-action, as these may require an altered tone to show any efficacy. On the other hand, animal disease state models that show some biological alterations also observed in a subpopulation of patients with depression will guide selection of the target population in clinical development later on.
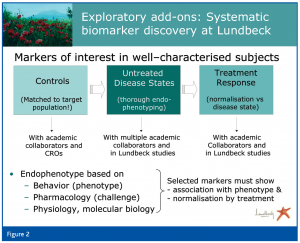
In view of the lack of consensus on biological markers for the majority of psychiatric disorders, without consideration of the subtypes of disorders, we have initiated exploratory studies together with clinical development teams and academic investigators around the world (Figure 3). The aim of these studies is to associate distinct biomarkers with a specific clinical phenotype. This can only be achieved if state-of-the-art technology is employed and the subjects, including both healthy and symptomatic volunteers (or patients), are thoroughly phenotyped using appropriate self- and physician-rated scales. The dimensional aspect of psychiatry opens up the possibility of exploring biomarkers in unsymptomatic (healthy) volunteers, in whom known and presumed risk factors for certain disorders are documented. To ensure the relevance of such exploratory analyses it is important to assess a large number of well-characterised subjects, confirm findings and employ sophisticated statistical approaches (e.g. pattern recognition).
Patterns identified in control subjects are used to generate hypotheses that are tested in acutely ill, but untreated, patients with different, well characterised clinical phenotypes. This approach is pursued with dependable clinical investigators who recognise the need to profile patients thoroughly at the symptom and context level. The drawback of the often small sample size in academic collaborations is offset by the detailed clinical information. This allows generation of hypotheses that may be tested in larger clinical trials. In the latter, the detailed clinical characterisation is again paramount to confirm subgroups that have been detected in the smaller studies. Larger clinical trials that examine treatment effects will then enable selection of biomarkers that are indicative of a specific disease state, and those that are associated with a good treatment response. These latter biomarkers, upon confirmation, can then be proposed as surrogate endpoints.
The importance of pattern analysis also for safety aspects is supported by the rising literature on toxicogenomics and attempts to associate classical toxicological markers with gene expression patterns (Ekins, 2006). Another aspect of pattern analysis with regard to safety and tolerability is that thorough pharmacodynamic profiling of drug candidates can also help to select target populations; for example, while activation of the stress axis may be an unwanted effect in some psychiatric patients, it may be a useful drug effect for other psychiatric populations. Such drug and population profiling will help to select the right drug in its optimal dose for the best patient population.
To ensure optimal use of biomarker data generated in such clinical studies, an iterative communication between discovery, clinical pharmacology and clinical development must be maintained. This process means to also actively address barriers to a successful integration, such as perceived risks and burden by the clinical teams, inappropriate funding and a late start to biomarker assessment in drug development. In our hands, biomarkers are part of the discovery process as soon as a drug candidate is nominated. Strictly speaking, our program starts even earlier, as it seeks to associate a panel of biomarkers with a specific disease biology even before a drug effect is explored. We are therefore assessing biomarkers systematically in healthy volunteers and untreated patients, to identify markers of vulnerability, resilience and different disease states, not confounded by treatment. At the same time, this approach provides useful information for mechanistic read-outs in animal models. We hope that this strategy will not only improve the predictive value of animal models for future drug development, but that it will also identify novel drug targets by determining resilience markers.
In psychiatry this seems particularly pertinent as clinicians have very limited tools to predict who will develop which disorder, who will respond to a given treatment and who will need long-term treatment for prevention of a new episode. As many academic investigators and clinicians are painfully aware of this problem, studies are being conducted around the globe to identify objective read-outs that help with the diagnosis as well as with selection of treatment. As academia is under pressure to publish, confirmation and reproducibility of results is not always a main focus of published work. For pharmaceutical companies, in turn, reproducible and reliable data are of utmost importance, if a development program is to be based on such data. Therefore, public-private collaborations can help to generate data that withstand the required scrutiny. Ultimately, the goal for all the stakeholders involved should be to move towards personalised medicine, aiming to have tools at hand that allow the selection of the right drug for the right patient at the right time.



References
1. Biomarkers Definitions Working Group (2001): Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69: 89-95.
2. Ekins S (2006): Systems-ADME/Tox: resources and network approaches. J Pharmacol Toxicol Methods 53: 38-66.
3. Insel TR, Scolnick EM (2006): Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry 11: 11-17.
4. Parker G (2004): Evaluating treatments for the mood disorders: time for the evidence to get real. Aust N Z J Psychiatry 38: 408-414.
5. Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al (2006): Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163: 28-40.




