A vision for the future
Posted: 24 March 2006 | | No comments yet
Due to the need for improvement in the cost structure and efficiency of the pharmaceutical industry, the introduction of NIR analytical techniques in combination with PAT applications is a promising opportunity for cycle time reduction and machine utilisation increase.
Due to the need for improvement in the cost structure and efficiency of the pharmaceutical industry, the introduction of NIR analytical techniques in combination with PAT applications is a promising opportunity for cycle time reduction and machine utilisation increase.
Due to the need for improvement in the cost structure and efficiency of the pharmaceutical industry, the introduction of NIR analytical techniques in combination with PAT applications is a promising opportunity for cycle time reduction and machine utilisation increase.
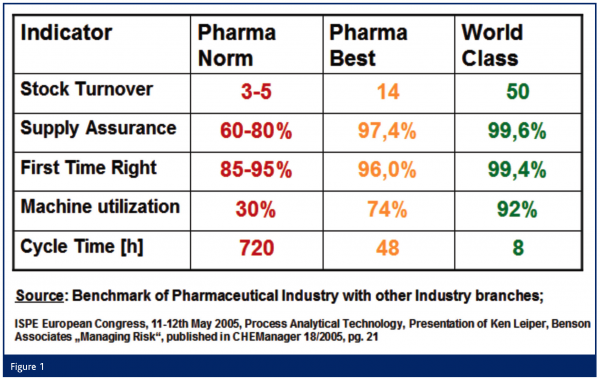
A main driver for the introduction of NIR analytical techniques for PAT applications results from the poor supply assurance situation and the first-time-right ratio of the pharmaceutical industry compared with other industries (Figure 1). Due to very low machine utilisation and extraordinarily long cycle times, stock turnover is much slower and therefore there is huge potential for improved cost efficiency in the pharmaceutical industry. PAT applications are a promising opportunity to reduce cycle time and improve machine utilization, thus reducing costs and machine downtime. In the field of PAT NIR is one of the most flexible options for implementation.
Typical applications
NIR spectroscopy is conventionally used for identity testing, determination of water content and active pharmaceutical ingredient (API) content or physical properties. Typical PAT applications of NIR spectroscopy can be found for blending, granulation, capsule filling, tabletting, coating and blister packaging.
The NIR principle was discovered in 1800 by Herschel. NIR Instruments have been developed since the 1950s, triggered by technological innovations (Siesler et al., Near Infrared Spectroscopy, Wiley-CH, 2002). Since the 1980s the development of high tech computation lead to the introduction of multivariate analysis methods which made it possible that NIR could be used for broader and more complex applications. First, stand-alone NIR instruments were introduced, but since the 1990s NIR has become a widely used analytical technique due to chemometrical methods, fiber optics and miniaturisation.
Regulatory authorities therefore introduced NIR spectroscopy in 1997 into the European Pharmacopoeia and in 2002 into the American Pharmacopoeia. Moreover, in August 2003 the FDA published a PAT Guidance that became official in September 2004 and enforced with this guidance further implementation of NIR applications.
The spectral range of Infrared radiation reaches from 800nm to 500µm with the following classification:
Far Infrared (FIR) 50µm – 500µm or 200cm-1 – 20cm-1
Mid Infrared (MIR) 2500nm – 50µm or 4000cm-1 – 200cm-1
Near Infrared (NIR) 800nm – 2500nm or 12500cm-1 – 4000cm-1
While FIR enhances molecule rotation, MIR and NIR enhance molecule vibrations. A molecular vibration is a combination of valence vibrations and deformation vibrations. Since a molecular bond behaves similar to an anharmonic oscillator acc. to Quantum theory, discrete energy states are enhanced. While MIR is able to enhance the molecular base vibrations, NIR reaches only the weaker overtones of molecular vibrations. The higher the overtone, the closer this overtone lies energetically to the next overtone above. Therefore, NIR radiation enhances an overlapping multitude of transitions and combinations of overtones of molecular vibrations. This results in overlapping of individual spectral absorption bands and thus in very broad spectral signals.
This is the reason why data evaluation of NIR spectral information is usually only possible by computational methods, so called chemometrics. According to Schrödinger’s Equation the excitation energy has to be increased with increase of the chemical bond strength and with decrease of the vibrating atomic mass. Accordingly, NIR radiation enhances overtones of C-H, O-H, N-H, S-H, C=O bonds which can be widely found in organic substances.
Chemometrical methods include multivariate analysis, e.g. distance measurements (Euclidic Distance, Mahalanobis Distance, Maximal Distance) and algorithms like PCA, SIMCA, PLSR, PCR and Cluster analysis. A good overview of theoretical background of chemometrics can be found in R. Kramer, Chemometric Techniques for Quantitative Analysis, Marcel Dekker, 1998 or in Chemometrics – A Practical Guide – K.R. Beebe, R.J. Pell, M.B. Seasholtz, John Wiley and Sons (1998). A helpful guideline for application of chemometrical methods is the PASG guideline (http://www.pasg.org.uk/ NIR/NIR.htm).
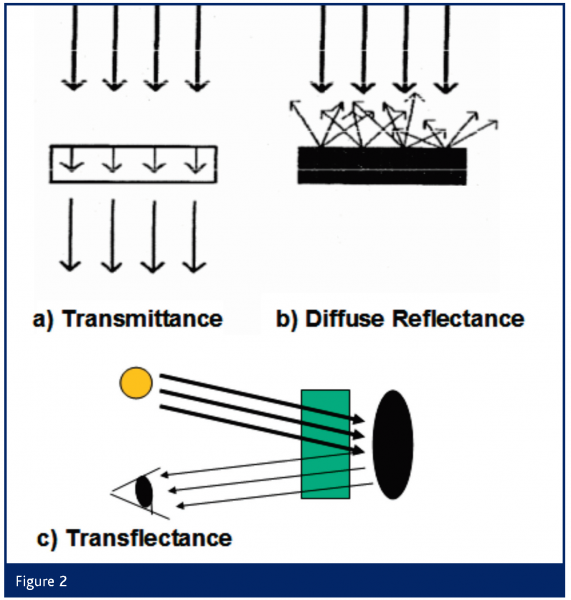
NIR measurements can be executed in three different ways (Figure 2): A) Transmittance: NIR radiation permeates the sample and is partially absorbed. B) Diffuse reflectance: NIR radiation interacts with an impermeable sample and is absorbed and diffusely reflected. C) Transflectance: NIR radiation permeates the sample and is reflected by a mirror or prism.
These three different NIR measurement techniques can be applied to a variety of substances. Liquids and solvents are usually analysed in transmittance in a cuvette, with a transmittance probe, a transmittance distance or a transflectance probe, whereas solida, creams etc. are measured in diffuse reflectance with a reflectance probe or an integration/Ulbricht sphere. Furthermore, add-ons such as autosamplers and tablet holders are available off-the-shelf. As detectors for the range of 1100-2500 nm in general standard Indium-Gallium arsenide (InGaAs) detectors or Lead sulfide (PbS) Semi-conductor detectors are used. In the broadly developed NIR spectrometer supplier market a variety of dispersive and Fourier Transformation (FT-) NIR spectrometers are commercially available. Different monochromator principles both with mobile mirrors (Grating refraction, Michelson / Polarisation interferometer) and immobile parts (Diode array system, Accusto-optical flexible Filter) are available.
What can be measured by NIR?
Liquids are analysed in transmittance. Here the Lambert Beer Rule applies with a direct proportional relation between absorption and concentration: A = e x conc x d ; A = log 1/T
Solids however are analysed in diffuse reflectance or transmittance. Solid materials have an unpredictable reflectance behaviour that is a combination of specular and diffuse reflectance and absorption, since the NIR beam has a penetration depth in sample material of 1-3 mm. Therefore, the concentration is roughly proportional to the logarithm of the inverse reflectance: log (1/R) ~ concentration. This is the reason why quantitative NIR measurements of Solida require a reference database which can be compared to unknown samples.
Due to the lack of organic molecular bonds the following materials are not detectable with NIR:
- Inorganic substances
- Heavy metals
- Metal
- Elements
- Gas (only in very large layer thickness, ca. 100m)
Advantages of NIR spectroscopy: It is a direct, non- destructive measurement technique with measurement times < 1min. After minimal sample preparation simultaneous testing of several characteristics is possible. Since water is the ideal absorber for NIR, measurements of aqueous solutions and humidity are very easy and accurate. Measurements directly through a glass window or plastic foil allow material to be analysed without unpacking or opening containers or process equipment. It is possible to decouple the measurement location and NIR spectrometer position thanks to fiber optics (up to several 100m). NIR is an easy technique that allows for delegation of measurements to staff directly at the manufacturing location or even on-line and touch free. Staff can easily be trained to handle NIR spectrometers; no lab analyst qualification is required. Sample handling is much easier compared to IR, ATR, Raman and HPLC.
A disadvantage of NIR is the need for application of a secondary method based on regression of primary data. Moreover, the complex set up of an NIR database, the impossibility to measure impurities and inorganic substances and the difficulty to apply NIR to molecules of elements with heavy mass is a limiting factor. If one is not interested in water, interferences may disturb the measurement. Fiber optics are also easily affected by mechanical stress, buckling and temperature, which might lead to glass fiber breakage and irreparable damage. Therefore careful handling of fiber optics has to be ensured.
Pharmaceutical rules and guidance on NIR
Due to increasing interest in NIR spectroscopy, several pharmaceutical rules and guidance sources are available. Firstly, the pharmacopoeias have already published detailed general chapters about NIR spectroscopy. Also, the EMEA Note for Guidance (http://www.emea.eu.int/)
‘Note for Guidance for NIR Spectroscopy in Pharmaceutical Industry and required Data for new Submissions and Re-registrations of the EMEA’ has been valid since August 2003 and describes in detail the European requirements of authorities with respect to NIR.
The European focus on NIR technology can be explained because it was introduced in the mid 1980s in response to EU GMP Guideline 5.30 “There should be appropriate procedures or measures to assure the identity of the contents of each container of starting material.”
Besides the regulatory authorities, other institutions have published helpful guidelines for NIR spectroscopy, listed below:
1) Overview of ASTM Guidelines:
E275-93 Performance of UV, VIS and NIR Spectrometers
E1790-00 NIR Qualitative Analysis
E1655-00 Infrared Multivariate Quantitative Analysis
E1944-98 Performance of FT-NIR-Spectrometers:
Level 0 and 1 Tests
E1791-96 Reflectance Factor for NIR Instruments using
hemispherical Geometry
D6122-01 Scope for PAT Applications
2) PASG ‘Guidelines for the Development and Validation of Near Infrared (NIR) Spectroscopic Methods’ (Oct. 2001)
Finally, the FDA Guidance for Industry ‘PAT – A Framework for Innovative Pharmaceutical Manufacturing and Quality Assurance’ (August 2003) further increased the interest in NIR applications as well as other analytical techniques.
This list of rules and guidance accounts for the increased interest in NIR technology, both from the pharmaceutical industry and regulatory authorities. The regulatory impact for pharmaceutical industry of these rules and guidance leads to more reliability, more legal security, but also more required documentation for registrations in EU. Due to the demonstrated analytical expertise of authorities in the NIR field, this guidance can be used as an official reference point for the introduction and application of NIR. It is now possible to refer to precedents. However, these publications all demonstrate the expectation of the authorities to participate in the technological development of NIR applications.
PAT application
PAT is a system for designing, analysing and controlling manufacturing through timely measurements (i.e. during processing) of critical quality and performance attributes of raw and in-process materials and processes with the goal of ensuring final product quality. The key principals are:
- Quality by Design
- Process Understanding
- Continuous Improvement and
- Real Time Release
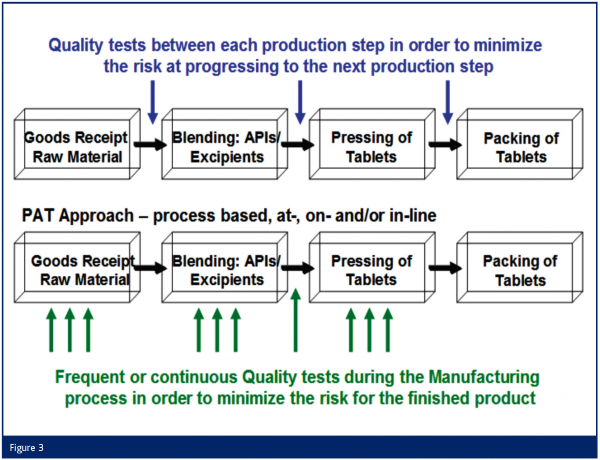
In the FDA guidance the following statement can be found: “This guidance is intended to describe a regulatory framework that will encourage the voluntary development and implementation of innovative pharmaceutical manufacturing and quality assurance. Working with existing regulations, the Agency has developed a new innovative approach for helping the pharmaceutical industry address anticipated technical and regulatory issues and questions.” This is clearly an indication of FDA’s expectation to evaluate applications like NIR for process controls according to the following scheme (Figure 3):
These PAT applications will not only lead to improved process understanding and higher process safety and product quality, but also shorter process lengths, a reduction of non conformance batches, laboratory and validation efforts and long-term also to parametric release. This is an opportunity for a win-win situation for both patients and pharmaceutical industry since the cost efficiency, cycle time and machine utilisation can be clearly improved by the PAT approach.
Finally, as a vision for the future the combination of PAT and quantitative NIR measurements can make real time release possible!