Dosing and weighing in the pharmaceutical laboratory
Posted: 7 March 2005 |
Dispensing of solids is still a demanding task in laboratory automation. The solubilisation of High Throughput Screening (HTS) libraries is one of the most challenging problems in this area, since millions of different substances have to be processed by the same technology.
Dispensing of solids is still a demanding task in laboratory automation. The solubilisation of High Throughput Screening (HTS) libraries is one of the most challenging problems in this area, since millions of different substances have to be processed by the same technology.
Dispensing of solids is still a demanding task in laboratory automation. The solubilisation of High Throughput Screening (HTS) libraries is one of the most challenging problems in this area, since millions of different substances have to be processed by the same technology.
The distribution and dosing of chemicals plays an important role in the chemical and pharmaceutical industry. Be it the addition of reagents in chemical production or the dosing of ingredients in pharmaceutical production, exact dosing and weighing is of utmost importance. At the same time, efficiency demands that these processes be automated and a variety of systems have been developed to this end.
In production facilities, batch-wise dosage of bulk chemicals is frequently integrated into the reaction process. Solid reaction educts are added to the reaction vessel, mostly via feeders equipped with vibratory devices, or feeders equipped with screws or spirals for a controlled powder flow. The correct amount of the solid substance can either be determined by a constant rate of powder flow or by the weight increase of the reaction vessel itself. Similar processes are used for the packaging of finished products before shipment.
Many studies on different kinds of feeders were carried out in order to find techniques that are best suited for dosage of different powders1. It was found that vibratory feeders with vibratory control have the broadest applicability for different powders2; however, each system must be optimised for the specific powder properties at hand. In general, all these systems have in common that they deal with a limited number of different materials.
Chemical process research is another area where automated addition of building blocks, catalysts or other reactants gains importance3. The optimisation of yield and side product profile of a chemical reaction often requires many experiments with only minor changes to the reaction conditions. Increasingly, this is achieved in a screening approach were automated reagent addition allows testing of, for example, a number of catalysts in a short time. Also in combinatorial and parallel synthesis, automated dosing of building blocks and reagents is used to speed up the preparation of libraries of related compounds4.
Automated weighing in drug discovery research
In drug discovery research, the highest level of automation is achieved in lead discovery. Here, millions of compounds are screened for lead structures that serve as starting points for medicinal chemistry. Large compound collections have been assembled not only by Big Pharma, but also by increasing numbers of biotech companies and academic research institutions. The preparation of screening solutions in a defined concentration from these millions of compounds is a daunting task, if automation cannot be brought to bear on this process.
Compound solubilisation for HTS poses a very challenging automation problem. Any automated weighing system cannot be optimised for a specific material, but must deal with many hundred thousands of different powders. Each material poses its specific challenges: solids that behave sand-like are very easy to dose, but tend to overshoot in weight, since they have a high flow rate. On the other hand, hygroscopic substances often become sticky. Solids with a low density and a small particle size are most affected by electrostatic charges and present problems even with a manual weighing process. The broad range of particle size distribution of substances makes it difficult to control the powder flow. Even the weather, especially the humidity, has dramatic effects on the weighing accuracy.
The amounts to be weighed are usually in the mg range and a high precision process is required. However, the process must also be fast, considering the number of samples that have to be dealt with. Therefore, a compromise between precision and speed must be struck. As a consequence of these challenges, only a few companies have moved to automate the solution preparation for HTS libraries beyond an IT-guided manual weighing station. When carried out manually, solution preparation is a people-intensive process with a limited throughput. However, as we will argue in the next section, a high capacity for solution preparation is a critical success-factor in Lead Discovery.
High throughput solution production critical for Lead Discovery
For many years, drug discovery groups have looked at miniaturisation, increased library size and assay throughput as ways to increase the productivity of their lead discovery efforts. Only too often this focus on quantity has led to compromises in the quality of the screening process and, inevitably, in the quality of the resulting data. High false positive rates are only one indicator of this situation. While false positives are a nuisance in themselves, adding to the bottleneck in cherry picking and hit validation, they point to a larger issue. New approaches in target finding, such as chemical genetics or systems biology, would like to draw on the wealth of data accumulated in research databases, only to find that large parts of the data do not meet quality requirements that would allow conclusions to be drawn from them.
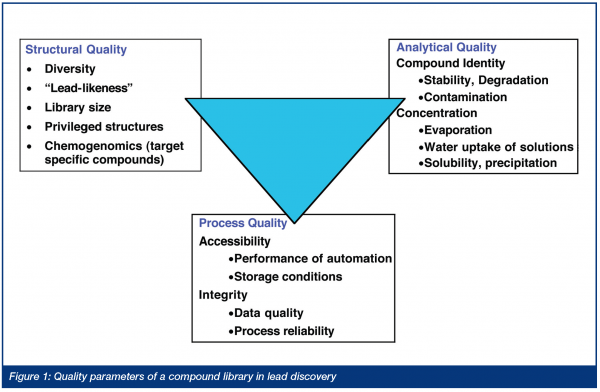
For these reasons, the quality of screening sets receives increasing attention, with companies looking both at the content and the analytical quality of their libraries5 (Figure 1).
However, even under ideal storage conditions, the quality of DMSO solutions will suffer over time6. The compounds should therefore be resolubilised at regular intervals. In a manual process, the weighing step is usually the bottleneck, because it is slow and labour intensive. Only the automation of the weighing step allows a lifecycle management for the screening library, with the possibility to remove undesired compounds in regular intervals. The process time for the introduction of new compounds to the screening set is also shortened. In view of the benefits of quality, automated weighing is a high priority in compound management.
Automated production at Novartis
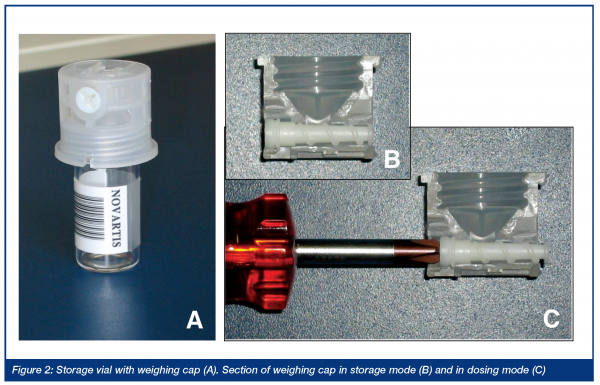
The first robot for automated weighing, the result of a collaboration with REMP (Switzerland), was made operational in 1997. The weighing mechanism is based on a specially designed cap for the storage vials. The cap consists of a funnel-like interior and is fitted with a screw-feeder for the dosing of powders (Figure 2).
Unlike similar systems, the screw-feeder is oriented horizontally and has two positions. In the storage position (B, Figure 2) the screw-feeder forms a tight seal and closes the vial. In the dosing position (C, Figure 2), turning with a screwdriver transports powder to the tip of the screw. At the end of a dosing cycle, the screw-feeder is pushed back into the storage position and, after turning the vial upright again, the screw-feeder is turned backwards to release residual material back into the vial.
While the general process is well established, it is the detail that makes it difficult. To achieve the desired performance, the dosing/weighing operation has to be completed within a few minutes. However, optimisation for speed will negatively impact the dosing accuracy. The screw-feeder is under control of the balance reading. Most balances are slow to deliver a stable value so that the dosing process is likely to be stopped too late. Second, the weight increase not only depends on the blade speed of the screw-feeder, but also on the particle size and granularity of the material. It was essential, therefore, to adjust the blade speed of the screw-feeder not on the weight increase, but on the gradient of the weight increase. Now powders that are flowing slowly will be dosed with a high initial blade speed, significantly reducing the overall time of the process. Powders that are flowing fast, on the other hand, will be dosed with a lower initial blade speed, resulting in a more accurate dosing result. Towards the end of the process, close to the target weight, a particle sensor serves to ensure that the screw-feeder stops after every added particle to allow the balance to stabilise its reading.
The performance of this system is remarkable even today: on a single balance more than 100,000 weighing operations have routinely been performed each year. However, with an input capacity of 352 vials, the robot had to be loaded more than once per day (Figure 3). The dissolution of the material in 96-well blocks and finally the production of cherry-picking tubes and 384-well plates occurred on separate modules, each step requiring a manual generation of production requests and registration of plates by the IT group.
In 2000, we set out to design a second generation system, this time in collaboration with SYSMELEC (Switzerland) and accelab (Germany). While the basic principle of weighing remains the same, the new system allows an input of 3000 vials (Figure 4). With two balances working simultaneously, the time spent on transport steps can be minimised and a rate of 1000 weighs per day is routinely achieved. Each compound is processed individually to a solution in a sealed tube. The tubes are assembled in racks and the solution is transferred to 96-well blocks. Compounds for which the target weight was not achieved can be taken out of the process, without holding up production. These vials, together with oily compounds not suited to automated weighing, are directed to manual weighing stations that are fully integrated into the process flow. After manual weighing, the product is fed back into the system. The 96-well blocks are transferred to a replication station, where cherry-picking tubes and 384-well plates are produced. These items are automatically registered in global databases and are ready for loading in the solution archives.
Alternative approaches to automated weighing
A very different approach to automated weighing was introduced in 1998 by ZINSSER-ANALYTICS (Germany)7. Here, the dosing of solid samples is performed with a calibrated volumetric powder pipette. Originally designed for applications in combinatorial chemistry, the volumetric powder pipette system can be used in all applications that demand solid sample dosing and solution preparation. Such volumetric pipettes are available with fixed volumes or with either manually adjustable or software controlled variable volumes. A range between 10ul and 255ul, corresponding to quantities from 10 to 500mg can be covered with the variable volume pipettes. The lowest amount that is reported with a fixed volume pipette is 1.5mg8. As this technology is based on a volumetric principle, the particle size distribution of the material is a major factor in the precision of this method.
Another approach to solid powder dosing is presented by Swiss company LOBOTECH9. This system combines a split septum cap for compound flasks or bottles with a microprocessor controlled mechanical gripper that rhythmically presses the septum. When the septum is pressed, the slit opens and a certain amount of powder falls into the target tube. A software algorithm governs the frequency of the mechanical gripper and thereby the amount of dispensed material. Depending on the sensitivity of the balance and on the properties of the material, amounts in the mg range can be distributed with this system, with a reported precision of 0.02 mg.
Conclusion
While there are several systems for automated dosing and weighing on the market, our experience shows that it takes a combination of optimised consumables, refined algorithms and sophisticated automation systems to meet the demands of compound management in the pharmaceutical industry. Dosing and weighing is still a challenge in laboratory automation with room for exciting new developments. Enabling technologies, such as those discussed here, continue to pave the way for innovative paradigms in drug discovery research.






Acknowledgements
The authors acknowledge the vision and contribution of Pietro Bollinger, Peter Maegli, Michel Girod, Pierre Acklin, Rene Amstutz and Otto Ramsebner.
References
- V. Kehlenbeck, K. Sommer, Powder Technol. 2003, 138, 51-56.
- G. I Tardos, Q. Lu, Advanced Powder Technol 1996, 7(1), 51-58.
- M. Harre, U. Tilstam, H. Weinmann, Organic Process Research & Development 1999, 3, 304-318.
- P. Josses, B. Joux, R. Barrier, J. R. Desmurs, H. Builliot, Y. Ploquin, P. Metivier, Adv. Lab. Autom. Robotics 1990, 6, 463-475.
- A. Schuffenhauer, M. Popov, U. Schopfer, P. Acklin, J. Stanek, E. Jacoby, Combinatorial Chemistry and High Throughput Screening 2004, 7(8), 771-781.
- U. Schopfer, C. Engeloch, J. Stanek, A. Schuffenhauer, E. Jacoby, P. Acklin, Combinatorial Chemistry and High Throughput Screening 2005, in press.
- W. Zinsser, O. Jenner, F. Payne, Buisiness Briefing: Pharmatech 2003, 1-3 (www.bbriefings.com).
- W. Zinsser, B. Jocksch, O. Jenner, Buisiness Briefing: Future Drug Discovery 2003, 1-3 (www.bbriefings.com).
- www.lobotechlbt.com




