Targeted therapies in lung cancer and Biomarkers
Posted: 16 December 2010 |
Despite innumerable clinical studies in the past three decades with lots of traditional chemotherapeutical drugs and drug combinations, survival in lung cancer has increased by far less than other entities. Research now focuses on inhibitors of tyrosine kinases which have been shown to have a central role in the development of lung cancer. However, as recent developments show, unselected use of those ‘targeted therapies’ is not always effective and may even be harmful to lung cancer patients if given at the wrong time or to the wrong patient. Biomarkers with predictive value will, in future, be of utmost importance for an individualised tumour tailored therapy. In this perspective, we describe the latest developments in EGF-R directed tyrosine kinase inhibitors and other targeted therapies. Additionally, the actual (limited) predictive role of biomarkers is discussed in this context and further directions are pinpointed.
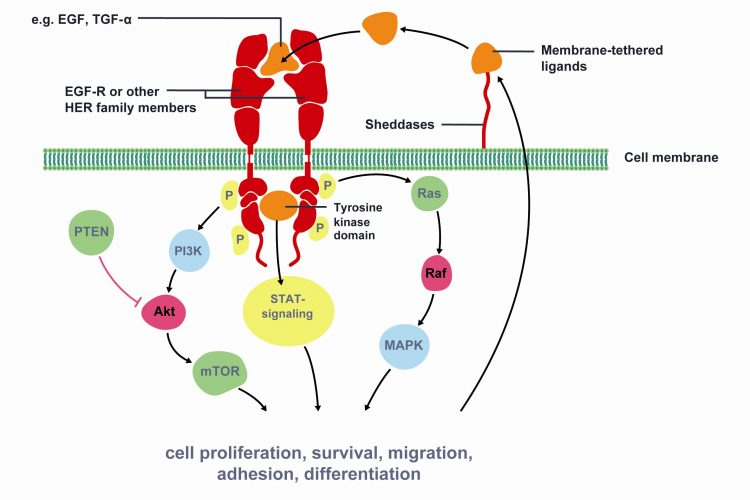

Figure 1 Key signaling pathways of Epidermal Growth Factor Receptor (EGFR). The epidermal growth factor receptor (EGFR) is a member of the human epidermal growth factor receptor (HER) superfamily of receptors comprising of four distinct however structurally similar tyrosine kinase receptors. Upon ligand binding (e.g. EGF, TGF-α) EGFR dimerises with another receptor and undergoes phosphorylation of its TK domain. Activated EGFR stimulates cell proliferation, survival, migration, adhesion and differentiation. EGFR is associated with increased or inappropriate signaling in NSCLC and is a key mediator of tumor progression. Activating mutations of the EGFR kinase domain result in ligand-independent activation of the pathway. Tyrosine kinase inhibitors, such as erlotinib and gefitinib, interfere with the kinase activity of the gene and prevent downstream signaling. Therefore, EGFR is an important target for NSCLC treatment. Modified from Gazdar et al22
Despite innumerable clinical studies in the past three decades with lots of traditional chemotherapeutical drugs and drug combinations, survival in lung cancer has increased by far less than other entities. Research now focuses on inhibitors of tyrosine kinases which have been shown to have a central role in the development of lung cancer. However, as recent developments show, unselected use of those ‘targeted therapies’ is not always effective and may even be harmful to lung cancer patients if given at the wrong time or to the wrong patient. Biomarkers with predictive value will, in future, be of utmost importance for an individualised tumour tailored therapy. In this perspective, we describe the latest developments in EGF-R directed tyrosine kinase inhibitors and other targeted therapies. Additionally, the actual (limited) predictive role of biomarkers is discussed in this context and further directions are pinpointed.
Lung cancer is the main cause of malignant tumour deaths in the world with more than 1.2 million cancer deaths per year and is divided in to small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). The latter accounts for approximately 80 per cent of lung cancer and will be the subject of this review. Prognosis of patients with lung cancer is worse in contrast to other tumour entities such as colorectal or breast cancer. With respect to tumour stage and therapy, the five-year survival rate differs between 14 per cent (all stages) and 67 per cent (after surgery with curative intent).
At least 40 per cent of the patients are diagnosed with metastatic disease. Beside best supportive care (BSC), a chemotherapy in palliative intent is recommended for patients with good or acceptable performance status. Using anti-tumour drugs, survival of those patients was improved from 2002 to 2009 with median survival times of 7.9 months to 11.3 months1,2. Usually a combination therapy with a cisplatin or carboplatin based back bone is used. Approved combination partners are taxans, cell-spindle inhibitors or antimetabolites. To date, the only approved antibody in combination therapy is bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor, leading to an increase of median survival of 12.3 months in clinically and histologically selected cases3. Because of the enormous range in effect, the type and severity of toxicity and the negative impact of quality of life between the substances, an individualised therapeutic approach would be more than desirable. However, to achieve this goal, validated predictive biomarkers must be known for prejudging the effect of the selected substance in each patient before starting the treatment.
A first step in this direction is now offered by the possibility of testing lung tumour patients due to an activating mutation of the epidermal growth factor receptor (EGF-R) in their primary tumours. Those patients with activating mutations can be effectively treated in an individualised manner with a tyrosine kinase inhibitor (TKI). TKIs are small molecules which can pass the basement membrane and act at the intracellular part of the receptor by inhibiting its phosphorylation and subsequent signal transduction. The receptor binding capacity for ATP is much decreased in tumours harbouring an activating mutation in the EGF-R gene and therefore competitive inhibition is in favour of the TKI4. Large prospective studies in both Caucasian and Asian NSCLC patients with EGF-R mutated tumours have concordantly shown response rates of 70 per cent in first-line palliative TKI based treatment with a median PFS of 9.5 and 14 months, respectively5,6. Therefore, palliative first line therapy of NSCLC patients exhibiting an activating EGF-R mutation in their tumours with the TKI gefitinib was approved in most European countries. At this year’s congress of the American Society of Clinical Oncology (ASCO), a study was presented comparing the TKI erlotinib with and without chemotherapy in palliative first-line treatment of non or only light-smoking patients with adenocarcinoma. There was no difference in progression free survival (PFS) and overall survival (OS)7. However, as expected, there was a significant improvement in both endpoints in patients with an activating mutation of the EGF-R. So far, so good.
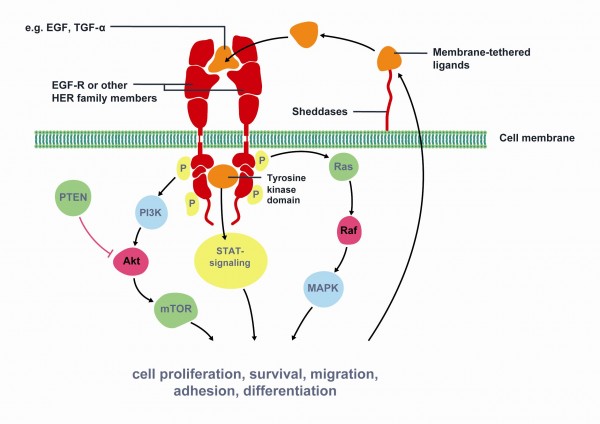

Figure 1 Key signaling pathways of Epidermal Growth Factor Receptor (EGFR). The epidermal growth factor receptor (EGFR) is a member of the human epidermal growth factor receptor (HER) superfamily of receptors comprising of four distinct however structurally similar tyrosine kinase receptors. Upon ligand binding (e.g. EGF, TGF-α) EGFR dimerises with another receptor and undergoes phosphorylation of its TK domain. Activated EGFR stimulates cell proliferation, survival, migration, adhesion and differentiation. EGFR is associated with increased or inappropriate signaling in NSCLC and is a key mediator of tumor progression. Activating mutations of the EGFR kinase domain result in ligand-independent activation of the pathway. Tyrosine kinase inhibitors, such as erlotinib and gefitinib, interfere with the kinase activity of the gene and prevent downstream signaling. Therefore, EGFR is an important target for NSCLC treatment. Modified from Gazdar et al22
Resistance to TKIs and overcoming it
However, there are not only activating mutations in the EGF-R gene, but also inhibiting ones can be found leading to resistance. So far, it is unclear if these resistance mutations occur during TKI therapy, or if they are already present at diagnosis. The most common mutation is the so-called T790M mutation, an inhibitory ‘one amino acid exchange’ leading to conformational changes in the ATP pocket making the TKI less able to bind8,9. A new generation of TKIs are now in clinical studies trying to overcome this kind of resistance by binding irreversibly to the EGF-R. Additionally, they inhibit further members of the HER receptor family, not only HER1 (=EGF-R). First results from phase II studies of such an irreversible TKI (PF299804) have been presented at this year’s congress of the ASCO. Results are promising with a PFS in the second or third line palliative therapy with a significant 12.4 weeks in comparison to 8.3 weeks with first generation TKI erlotinib10. Biomarker analysis revealed a positive predictive value concerning progression free survival for PF2999804 in k-ras wildtype and combined k-ras and EGF-R wildtype tumours with a HR of 0.52 and 0.62, respectively. However, the case numbers available for molecular analyses in this clinical trial were very small and reveal confirmation in a prospective phase III study.
Another recently identified inhibitory mechanism during TKI therapy occurs due to amplification of the c-met gene. C-met is the receptor of the hepatocyte growth factor (HGF) and de novo amplification of this pathway can circumvent inhibition of the EGF-R pathway. Therefore, it might be a reasonable consequence to block both pathways for better tumour control. Proof of principal was demonstrated at this year’s ASCO. In a palliative second line setting, the TKI erlotinib was tested against a combination of the TKI and the c-met inhibitor ARQ 197-20911. It could be shown in this randomised placebo-controlled phase II study that the combination is indeed superior to the TKI alone with a median progression free survival (PFS) of 16.1 and 9.7 weeks, respectively (adjusted HR 0.68). The results became more intriguing when the subgroup of non-squamous cell carcinoma was analysed (HR 0.61). Additionally, it was surprising that biomarker analysis revealed a benefit for the combination therapy in patients with k-ras mutation (HR 0.18) and EGF-R wild type (HR 0.70). However, the number of analysed tumour cases were very low.
Limited role of the EGF-R directed therapy in the adjuvant setting
More than astonishing and yet not completely understood are the results of a large prospective randomised phase III trial where a TKI was used in the adjuvant setting. In the BR.19 study gefitinib was given in comparison to placebo after curative resection of NSCLC stages I to III12. First of all, the rate of activating EGF-R mutations in this study was very high with 21 per cent of tested tumours. Therefore, it could be assumed that at least those patients should have demonstrated a benefit from the adjuvant TKI treatment. However, the opposite was true. The TKI treatment was not associated with an increased survival time, whether for patients with wild type tumours nor for those with an activating mutation. Even more, there was a trend for a decreased survival for the TKI cohort in comparison to the placebo (5.1 years with placebo versus 3.7 years with TKI mOS in EGF-R mutated patients; HR 1.58). The authors concluded that gefitinib did not remain significant but there was a trend suggesting it may be harmful (p=0.097). One might argue that the study was underpowered as accrual was stopped prematurely after N=503 of planned N=1160 patients. Other hypotheses address potential molecular differences between the primary (removed) tumour and the (remaining) tumour cell spread. At the moment, all these hypotheses have only speculative character. Therefore, the results of the ongoing RADIANT study, a phase III randomised clinical trial with planned N=945 patients where the TKI erlotinib is tested in the adjuvant setting in comparison to placebo are eagerly awaited.
Effectiveness of the TKI erlotinib in EGF-R wild type tumours
On the one hand, EGF-R mutations were not of predictive value in the treatment with the TKI gefitinib in the adjuvant setting, on the other hand, the EGF-R TKI erlotinib may have an effect not only on mutated cases but also on wild type tumours. The latter was initially demonstrated in the BR.21 study. In this phase III clinical trial, erlotinib was tested against best supportive care (BSC) in N=731 patients with progression to at least one palliative treatment and not tested for EGF-R mutation13. It could be shown that the patients randomised in the erlotinib arm had a significant better median overall survival in contrast to those in the BSC arm (HR 0.7; p<0.001). In a retrospective analysis of the EGF-R mutation analysis in N=197 tumour samples, survival after treatment with erlotinib was not influenced by mutation status14.
Further evidence came from the SATURN study. In this prospective phase III clinical trial erlotinib (or placebo) was given as a maintenance therapy to N=889 patients after at least ‘stable disease’ had been achieved by a platinum based first line chemotherapy15. Overall survival was significantly prolonged with erlotinib versus placebo for the intention to treat population (HR 0.81; p=0.0088) and even in the group of N=388 patients, whose tumours did not harbour activating EGF-R mutations (HR 0.77; p=0.0243). In line with evidence are also data from the TOPICAL study, presented at this year’s ASCO. In this prospectively randomised phase II study the TKI erlotinib was compared to BSC in patients > 75 years16. It could be shown in the multivariate analysis that women had a significant survival benefit of 5.3 months with the TKI in comparison to BSC (4.3 months; p=0.025). Again, the presence of an EGF-R mutation was not crucial for the benefit, as 98 per cent of the women expressed the wild type receptor. In conclusion, it is not clearly understood so far why erlotinib is effective in at least some EGF-R wild type tumours and it can be hypothesised that pathways other than the well known RAS-RAF-MAPK signal transduction might become inhibited from this substance.
Pathways of derived targeted therapies – often not fully understood
Further evidence for the discrepancies between our actual understanding of molecular pathways and clinical behaviour comes from NSCLC studies with the EGF-R antibody cetuximab. While in colorectal cancer a k-ras mutation was shown to be a negative predictor of response to this antibody driven therapy, this could not be confirmed for lung cancer. In the FLEX study, a prospective randomised phase III trial, a palliative first line therapy consisting of cisplatin, vinorelbine and cetuximab was compared with chemotherapy alone1. While the main endpoint was met and showed a significant increase in OS (11.3 months versus 10.1 months, respectively; HR 0.871), there was no difference between k-ras mutated and wild type tumours. It was supposed that either antibody derived cell cytotoxicity (ADCC) or other mechanisms may be involved in NSCLC circumventing the RAS-RAF-MAPK signal transduction pathway. However, to date the exact mode of action is not understood.
Besides the EGF-R pathway, there are other pathways known from experimental data to be involved in lung cancer pathogenesis. One of those is the insulin-like growth factor receptor (IGF-R) pathway. Preclinical and early clinical data with an IGF-R inhibitor (figitumumab) were promising and therefore, the results of a prospective phase III study combining the antibody with platinum-based chemotherapy (versus chemotherapy alone) was eagerly awaited at this year’s ASCO. However, the results were disappointing17. The main endpoint OS was not met. Even more, there was a trend towards a better survival in the ‘chemotherapy only’ arm. Interestingly, free IGF-1 levels seem to play some predictive role as patients with levels below 1ng/ml had an even worse survival rate and more toxicity in the ‘figitumumab’ arm. Again, this molecular phenomenon is not understood in detail now. Similar results were obtained with another targeted therapy focused against the TRAIL receptor. A randomised phase II study with an antibody against this receptor in combination with chemotherapy did not show any difference in terms of progression free survival and overall survival (in comparison) to chemotherapy alone18. Biomarker analyses for this trial have not been presented.
Further directions in biomarker analysis
Future directions in lung cancer therapy have to focus on predictive biomarkers, developed by high throughput retrospective molecular analyses, e.g. gene expression analyses from large trials and then have to be validated in prospective studies. Another possibility is the research towards mutations, amplifications or fusion molecules in lung tumours and the development of targeted therapies against them. A successful example of the later strategy is the development of Crizotinib. This small molecule targets the anaplastic lymphoma kinase (ALK), which was recently discovered to be an oncogenic driver in about five per cent of NSCLC19. Latest results of a phase I/II study in heavily pre-treated metastatic lung cancer patients showed an objective response rate of 57 per cent. After six months, the progression free survival was 72 per cent, the median progression free survival was still not reached. Toxicities in summary were low and focused on the gastrointestinal tract. A phase III trial with this promising target is underway.
As more and more genes will have to be analysed, limited tumour material will soon run out. Especially in the metastatic situation, where no surgery takes place and diagnosis is often made from small biopsies or even from pleura effusion cytology, tumour cells are often quite rare and after immunostaining for histological diagnosis, hardly any material is left for biomarker analyses. In this situation, further strategies have to focus on analytic techniques, where DNA and RNA of only a few cells are sufficient for expression or mutation analyses, e.g. using silica coated magnetic particles20. Additionally, circulating tumour cells in blood might be an alternative in generating sufficient tumour yield. Recently, it could be shown that both activating EGF-R mutations and the inhibitory T790M mutation could be detected in blood, both corresponding to the mutations detected in the same patients’ primary tumour21. In summary, as personalised tumour tailored therapy will be a mainstay of future lung cancer treatment, biomarkers with both predictive and prognostic value are of utmost importance and every clinical trial should incorporate at least a secondary molecular based endpoint.
References
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373(9674):1525-31
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346(2):92-8
- Sandler A, Gray R, Perry MC, et al. Paclitaxelcarboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355(24):2542-50.
- Carey KD, Garton AJ, Romero MS, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res 2006;66(16):8163-71
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361(10):947-57
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361(10):958- 67.
- Janne PA, Wang XF, Socinski MA, et al. Randomized phase II trial of erlotinib (E) alone or in combination with carboplatin/paclitaxel (CP) in never or light former smokers with advanced lung adenocarcinoma: CALGB 30406. Proc Am Soc Clin Oncol 2010;28(15s):abstr. 7503
- Eck MJ, Yun CH. Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell lung cancer. Biochim Biophys Acta; 1804(3):559-66
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105(6):2070-5
- Boyer MJ, Blakchall FH, Park K, et al. Efficacy and safety of PF299804 versus erlotinib (E): A global, randomized phase II trial in patients (pts) with advanced non-small cell lung cancer (NSCLC) after failure of chemotherapy (CT). Proc Am Soc Clin Oncol 2010;28(18s):abstr LBA7523
- Schiller JH, Akerley WL, Brugger W, et al. Results from ARQ 197-209: A global randomized placebo-controlled phase II clinical trial of erlotinib plus ARQ 197 versus erlotinib plus placebo in previously treated EGFR inhibitor-naive patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 2010;28(18s):abstr LBA7502
- Goss G, Lorimer I, Tsao MS, et al. A phase III randomized, double-blind, placebocontrolled trial of the epidermal growth factor receptor inhibitor gefitinb in completely resected stage IB-IIIA non-small cell lung cancer (NSCLC): NCIC CTG BR.19. Proc Am Soc Clin Oncol 2010;28(18s):LBA7005
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated nonsmall- cell lung cancer. N Engl J Med 2005;353(2):123-32
- Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer – molecular and clinical predictors of outcome. N Engl J Med 2005;353(2):133-44
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebocontrolled phase 3 study. Lancet Oncol 2010;11(6):521-9
- Lee S, Rudd RM, Khan I, et al. TOPICAL: Randomized phase III trial of erlotinib compared with placebo in chemotherapynaive patients with advanced non-small cell lung cancer (NSCLC) and unsuitable for first-line chemotherapy. Proc Am Soc Clin Oncol 2010;28(18s):abstr. 7504
- Jassem J, Langer C, Karp DD, et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 2010;28(18s):abstr. 7500
- von Pawel J, Harvey JH, Spigel DR, et al. A randomized phase II trial of mapatumumab, a TRAIL-R1 agonist monoclonal antibody, in combination with carboplatin and paclitaxel in patients with advanced NSCLC. Proc Am Soc Clin Oncol 2010;28(18s):LBA 7501
- Bang YJ, Kwak EL, Shaw AT, et al. Clinical activity of the oral ALK inhibitor PF-02341066 in ALK-positive patients with non-small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 2010;28(18s):abstr. 3
- Darb-Esfahani S, Wirtz RM, Sinn BV, et al. Estrogen receptor 1 mRNA is a prognostic factor in ovarian carcinoma: determination by kinetic PCR in formalin-fixed paraffinembedded tissue. Endocr Relat Cancer 2009;16(4):1229-39
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008;359(4):366-77
- Gazdar AF. Personalized medicine and inhibition of EGFR signaling in lung cancer. N Engl J Med 2009;361(10):1018-20
About the Authors
Wolfgang Brueckl is an assistant professor of internal medicine at the Friedrich-Alexander University of Erlangen, Germany. Additionally, he is the senior consultant of Thoracic Oncology at the Nuremberg Lung Cancer Centre. After completing his MD at the Ludwig-Maximilians-University of Munich with summa cum laude he moved to the University of Erlangen, where he received a PhD in Molecular Oncology and specialised in Internal Medicine and Respiratory Medicine. His main clinical focus is the field of Thoracic Oncology. He is a principal investigator of numerous promising phase I-IV clinical studies. Furthermore, Professor Brueckl is a member of several advisory boards of international pharmaceutical companies. His research is focused on predictive and prognostic markers in lung cancer. Early research involved DNA repair mechanisms, microsatellite instability and microarray experiments. His current research is aimed at molecular marker based individualised tumour tailored therapy. He has published over 50 original publications in international peer-reviewed scientific journals and holds four patents in the field of prognostic markers in oncology. Contact the author: [email protected]
Joachim H. Ficker is a professor for internal medicine and head of the Third Medical Department / Department for Respiratory Medicine at the Klinikum Nuernberg, Germany. This department is one of the largest clinical units for respiratory medicine in Germany. He is the Director of the Nuernberg Lung Cancer Centre. This centre of excellence is accredited and recommended by the German Cancer Society (Deutsche Krebsgesellschaft) and was the first of its class established in Germany. He was one of the main authors responsible for the German S-3 guidelines for the diagnosis and therapy of lung cancer in Germany, published by the German Respiratory Society (DGP) and the German Cancer Society (Deutsche Krebsgesellschaft) in 2010.
Besides thoracic oncology, Professor Ficker has specialised in Sleep Medicine, where he has published more than 100 scientific articles. Additionally he (co-)authored several scientific books in sleep medicine. For many years, Joachim Ficker has been frequently invited as a speaker or chair of many nationwide and internal scientific conferences and he will be the congress president of the German Respiratory Society (DGP) Congress in 2012. He is a member of numerous advisory boards and reviewer of several peer-reviewed scientific journals in the field of Respiratory Medicine, Sleep Medicine and Thoracic Oncology.
Thomas Mundel is Medical Manager Oncology at Roche Pharma AG. He is the disease area scientific and medical expert for lung cancer, and his focus is targeted therapies, especially in non small cell lung cancer (NSCLC). Before joining Roche in 2009, he was Medical Advisor at Janssen-Cilag. Prior to this he was a scientist and research associate at the Division of Matrix Biology, Beth Israel Deaconess Medical Center, Harvard Medical School in Boston where he worked in the laboratory of Dr. Raghu Kalluri on collagen type IV metabolism. During this time he could show the first successful treatment of genetic kidney disease in a mouse model for human Alport syndrome with bone marrow-derived stem cells via bone marrow transplantation. He could also establish type IV collagen α6 chain-derived non-collagenous domain 1 (α6(IV)NC1) as a new endogenous inhibitor of angiogenesis and tumor growth. Thomas received his MD/PhD degree from the Ruprecht-Karls- University Heidelberg, and for this work he was awarded the Wolfgang-Bargmann-Preis of the German Anatomical Society. Clinically he specialised in general surgery at the university hospital Muenster, Germany, with focus on oncological surgery. He has co-authored numerous peer-reviewed publications.




