Pulmonary inhalation aerosols for targeted antibiotics drug delivery
Posted: 16 February 2011 |
Targeted pulmonary drug delivery of antibiotics by inhalation aerosols can play significant roles in the treatment of cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD) and in other pulmonary diseases where chronic airway infections exist. Direct administration to the lung as targeted pulmonary inhalation aerosol delivery is uniquely able to provide for high dose levels of drugs at the target site of action without systemic side effects. This review presents an overview of pulmonary inhalation aerosols, types of inhalation aerosols, aerosol formulation additives and present current research in the targeted pulmonary drug delivery of antibiotics for the treatment of pulmonary infections. Clinical trials of antibiotic inhalation aerosols are also discussed.
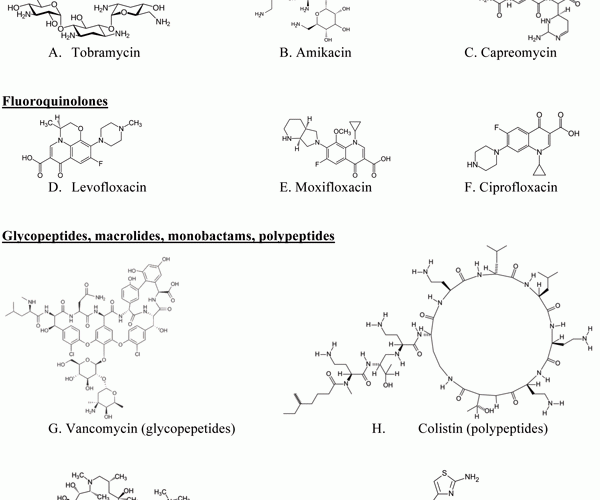

Figure 1 Examples of chemical structures of antibodies for targeted pulmonary inhalation aerosol
Targeted pulmonary drug delivery of antibiotics by inhalation aerosols can play significant roles in the treatment of cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD) and in other pulmonary diseases where chronic airway infections exist. Direct administration to the lung as targeted pulmonary inhalation aerosol delivery is uniquely able to provide for high dose levels of drugs at the target site of action without systemic side effects. This review presents an overview of pulmonary inhalation aerosols, types of inhalation aerosols, aerosol formulation additives and present current research in the targeted pulmonary drug delivery of antibiotics for the treatment of pulmonary infections. Clinical trials of antibiotic inhalation aerosols are also discussed.
Targeted delivery of drugs to the lung is highly desirable as the main advantages include reduced systemic side effects and higher dose levels directly at the site of drug action. This administration can be particularly useful for patients with chronic pulmonary infections and other pulmonary diseases, such as cystic fibrosis (CF), asthma or lung cancer1.
In the case of antibiotic drugs, the slow development of new classes of antimicrobials for the treatment of resistant bacterial infections has necessitated the development of alternative strategies in delivery. Targeted pulmonary delivery of antibiotics has gained increasing attention for the treatment of pulmonary infections, particularly chronic pulmonary infections in CF patients. Aerosol antibiotic administration can produce higher local concentrations of a drug that is far above those that can be obtained with conventional dosing and higher con – centrations can be applied to take advantage of concentration-dependent killing effects of antibiotics2-6.
In a recent report2,7-9, there were significant differences between aerosolised and intra – venous drug delivery of antibiotics, related to peak concentrations of serum and sputum. With aerosolised delivery of tobramycin, peak sputum concentrations were up to a maximum of 1,200 times higher than peak serum concentration, which is less than occurs with intravenous delivery. Delivery of effective local drug concentrations to the infection site via pulmonary inhalation aerosol decreases the risk of systemic side effects seen with long-term use of oral or intravenous antibiotic therapy10,11.
Currently, there are two U.S. FDA-approved antibiotics for inhalation. Tobramycin solution for inhalation12 (TOBI® 300 mg/5 mL, Novatis pharmaceutical Corp.) is approved with indication that management of CF patients with P. aeruginosa and lyophilised aztreonam for inhalation13 (CAYSTON® 75mg/vial, Gilead Science Inc.) is monobactam antibacterial, indicated to improve repiratory symptoms in cystic fibrosis patients with P. aeruginosa. The development of aerosol antibiotics has been particularly important for treatment in chronic infections in patients with CF. In these patients, the loss of a hydrated pericellular epithelial surface in the lung results in defective mucociliary clearance and ultimately chronic infection, most notably because of P. aeruginosa8.
Overview of pulmonary inhalation aerosols: type
Pulmonary inhalation aerosols can be divided into three principal categories: nebulisers, pressurised metered-dose inhalers (MDIs) and dry powder inhalers (DPIs), each class with its unique advantages and disadvantages14. Aerosolised tobramycin and aztreonam are approved as inhalation solutions by nebulisation. Most nebulised formulations fall into two categories: solutions containing a drug that is typically dissolved in saline (or occasionally in another solvent or cosolvent with alcohol) or drug suspensions containing a drug that is not soluble in saline or another solvent15-17. In MDIs, the drug is either suspended or dissolved in a propellant that is pressurised. Release of a metered volume of drug in propellant through a control valve causes a high velocity aerosol with expansion and evaporation of propellant.
DPIs combine powder technology with device design in order to disperse dry particles as an aerosol in the patient’s inspiratory airflow18,19. The patient quickly breathes in the powder, which is dispersed as aerosolised particles without chemical propellants. Therefore, a balance between the design of an inhaler device, drug formulation and the inspiratory flow rate of patient is required20,21.
Additives
Additives have an important role in the development of new formulations for pulmonary inhalation aerosols with modification of physicochemical properties of drug particles and their aerodynamic behaviour. There are few additives approved by the US FDA22 for pulmonary administration (Table 1). The additives in a formulation are generally in the range of 60–99 per cent (w/w) and may decrease the dose of active drug that can be administered. This may cause difficulties in the case of drugs such as antibiotics that are active at relatively high doses or some peptides and proteins with low pulmonary bioavailability23.
Table 1. The list of additives for U.S. FDA approved drug products as pulmonary inhalation aerosol
| Inactive ingredient | Dosage form | CAS number* | Maximum potency |
| Acetone sodium bisulfite | Solution | 540921 | 0.5 % |
| Acetylcysteine | Solution | 616911 | 0.5 % |
| Air | Gas | 132259100 | – |
| Alcohol | Aerosol, metered | 64175 | 95.89 % |
| Aerosol, spray | 64175 | 33 % | |
| Solution | 64175 | 25 % | |
| Alcohol, dehydrated | Aerosol, metered | 64175 | 34.54 % |
| Ammonia | Liquid | 7664417 | – |
| Ascorbic acid | Aerosol, metered | 50817 | 95.95 % |
| Aerosol, spray | 50817 | 0.1 % | |
| Solution | 50817 | 1.02 % | |
| Benzalkonium chloride | Solution | 8001545 | 20 % |
| Carbon dioxide | Gas | 124389 | 95 % |
| Cetylpyridinium chloride | Aerosol, metered | 6004246 | – |
| Chlorobutanol | Inhalant | 57158 | – |
| Chlorobutanol | Solution | 57158 | 0.5 % |
| Citric acid | Aerosol, metered | 5949291 | 2.00E-04 % |
| Inhalant | 5949291 | – | |
| Solution | 5949291 | 0.44 % | |
| D&c yellow no. 10 | Solution | 8004920 | – |
| Dichlorodifluoromethane | Aerosol, metered | 75718 | 74.02 % |
| Inhalant | 75718 | – | |
| Dichlorotetrafluoroethane | Aerosol, metered | 76142 | 51.12 % |
| Edetate disodium | Solution | 6381926 | 5 % |
| Edetate sodium | Solution | 64028 | 0.02 % |
| Fd&c yellow no. 6 | Solution | 2783940 | – |
| Fluorochlorohydrocarbons | Aerosol, metered | – | – |
| Aerosol, spray | – | – | |
| Glycerin | Solution | 56815 | 7.3 % |
| Hydrochloric acid | Aerosol, metered | 7647010 | 1.72 % |
| Solution | 7647010 | 3.5 % | |
| Solution, for inhalation | 7647010 | – | |
| Lactose | Capsule | 63423 | 20 mg |
| Capsule, hard gelatin | 63423 | 25 mg | |
| Powder | 63423 | 9 % | |
| Lecithin | Aerosol, metered | 8002435 | 2.00E-04 % |
| Lecithin, hydrogenated soy | Aerosol, metered | – | 0.28 % |
| Lecithin, soybean | Aerosol, metered | 8030760 | 0.1 % |
| Menthol | Aerosol, metered | 89781 | 0.05 % |
| Methylparaben | Solution | 99763 | 0.07 % |
| Nitric acid | Aerosol, metered | 7697372 | 1.67 % |
| Nitrogen | Gas, for inhalation | 7727379 | – |
| Solution | 7727379 | – | |
| Norflurane | Aerosol, metered | 811972 | 7.5 % |
| Oleic acid | Aerosol, metered | 112801 | 0.26 % |
| Inhalant | 112801 | 0.16 % | |
| Propylene glycol | Solution | 57556 | 25 % |
| Propylparaben | Solution | 94133 | 0.03 % |
| Saccharin | Aerosol, metered | 81072 | 0.11 % |
| Saccharin sodium | Aerosol, metered | 6155573 | 0.04 % |
| Solution | 6155573 | – | |
| Sodium bisulfate | Solution | 7681381 | 0.01 % |
| Inhalant | 7631905 | 0.07 % | |
| Solution | 7631905 | 0.2 % | |
| Sodium chloride | Inhalant | 7647145 | – |
| Solution | 7647145 | 3.16 % | |
| Solution, for inhalation | 7647145 | – | |
| Sodium citrate | Inhalant | 6132043 | – |
| Solution | 6132043 | 0.6 % | |
| Sodium hydroxide | Aerosol, metered | 1310732 | – |
| Solution | 1310732 | 8 % | |
| Sodium metabisulfite | Solution | 7757746 | 1 % |
| Sodium sulfate anhydrous | Solution | – | 0.02 % |
| Sodium sulfite | Solution | 7757837 | 0.1 % |
| Sorbitan trioleate | Aerosol, metered | 26266580 | 0.06 % |
| Sulfuric acid | Solution | 7664939 | 12.5 % |
| Thymol | Liquid | 89838 | 0.01 % |
| Trichloromonofluoromethane | Aerosol, metered | 75694 | 33.83 % |
| Inhalant | 75694 | – |
Current research issues of antibiotics inhalation aerosols based on targeted pulmonary drug delivery
Various antibiotics have been investigated for pulmonary inhalation aerosol for targeted drug delivery, including aminoglycoside, fluoroqunolone, glycopeptide, macrolide, monobactam and polypeptide. The chemical structures of antibiotics for targeted pulmonary inhalation aerosol are shown in Figure 1. The US FDA has approved inhalation aerosol formulations of amyloglycoside (tobramycin) and monobactam (aztreonam). There is considerable research occurring on lab- and pilot-scale for the novel targeted pulmonary delivery formulations of antibiotics that provide an increase in lung deposition efficiency and an optimisation of drug release profile in the lung.
Aminoglycosides: tobramycin, capreomycin, amikacin
Tobramycin (Figure 1A) solution for inhalation has been shown to have the potential limitation that the delivery of the drug from a nebuliser device can be inefficient i.e. ~ 88 per cent of the dose placed in the nebuliser can be lost during administration. Additionally, the inhaled dose by nebulised solutions is often limited by a drug’s solubility. Drug loss during exhalation and drug solution trapped within the nebuliser all account for the drug loss24.
In 2003, Newhouse et al. evaluated the efficiency and reproducibility of a tobramycin Pulmosphere® formulation, by dry powder inhalation, in healthy volunteers25. They reported that 150 milligrams of tobramycin DPI could be enough to be delivered to the lung and the systemic circulation using DPI as with the nebulised tobramycin solution for inhalation in approximately one-tenth of the time. This was subsequently confirmed in a recent pharmacokinetic study by Geller et al.26 and Novartis pharmaceutical Corp. has been trying a clinical research study phase III to compare tobramycin DPI formulation with tobramycin solution for inhalation (TOBI) in patients with CF.
Further improvements in tobramycin DPI delivery have been reported by Pilcer et al.27. In this study, the authors developed and evaluated a lipid-coated tobramycin dry powder formulation that had particularly high lung deposition. Pilcer et al.28 prepared tobramycin DPI formulations containing high drug concentrations with very low levels of additives by a spray dried method, and resulted in very high lung deposition with fine particle fraction (FPF) values of at least 68 and 53 per cent of the nominal dose for the lipidcoated and uncoated tobramycin formulations through scintigraphic and pharmacokinetic evaluations. Parlati et al.29 presented that spray dried powders of tobramycin containing sodium stearate (NaSt) and showed that the presence of the NaSt fatty acid (salt form) improves aerosolisation efficiency and stability of a tobramycin DPI formulation.
Capreomycin (Figure 1C), another aminoghlycoside antibiotic for antiinfective/ anti-tuberculosis has been investigated for the treatment of multi-drug resistant tuberculosis through targeted pulmonary delivery of the DPI formulation30. They reported that particles for DPI manufactured from a capreomycin solution (50 per cent ethanol) containing 80 per cent capreomycin sulphate and 20 per cent l-leucine were evaluated through in vivo lung deposition test of guinea pigs using a custom-designed dry powder-dosing chamber and they found that lung deposition concentrations of capreomycin DPI in guinea pigs though inhalation administration was higher than that of intravenous administration.
Amikacin (Figure 1B) inhale®31 solution is also being developed by collaboration with Novartis pharmaceutical Corp. and Bayer for treatment of gram negative pneumonia. This formulation can be possibly used as a hand-held ‘off-vent’ device for patients no longer requiring breathing assistance. Liposomal amikacin sulfate DPI has been developed with a sustained release effect in the lungs for treatment of CF. They reported that fine particles of lactose had a good effect on enhancement of lung deposition of liposomal dried powder formulation (LDPF) of amikacin sulfate32.
LDPF of tobramycin33 and gentamicin34 were reported as an alternative choice of treatment of P. aeruginosa infection in the lungs associated with CF. The liposomally encapsulated drugs showed sustained and controlled release at the site of action; hence showed better control of the infection and bacterial killing.
Fluoroquinolones: ciprofloxacin, levofloxacin
Recently, Adi et al.35 described preparation of co-spray dried DPI formulation combining doxycycline and ciprofloxacin (Figure 1F), effective fluoroquinolones antibiotics for gram-negative and gram-positive bacteria, and showed that co-spray dried microparticles of doxycycline and ciprofloxacin, were physically more stable than conventional single spray dried antibiotic and had improved aerodynamic properties of DPI formulations. Furthermore, the spray drying process did not affect the antibacterial activity of the drug. Adi et al.36 prepared antibiotic formulations containing ciprofloxacin and doxycline with PVA to control the release profile. All formulations had similar particle size distributions, thermal stability, acceptable aerosol performance and controlled release profiles. Tsifansky et al.37 reported combined formulation of ceftazidime and ciprofloxacin, containing dipalmitoylphosphatidylcholine (DPPC), albumin and lactose for the targeted pulmonary drug delivery system.
Zhao et al.38 reported spherical particles of ciprofloxacin using combination of liquid antisolvent precipitation method and spray drying method for pulmonary inhalation aerosol with optimised aerodynamic properties. And Novartis, in collaboration with Bayer, have been developing Cipro Inhale®, a targeted DPI formulation of ciprofloxacin for CF infections using a hand-held DPI inhaler31. It has been reported that spray-freeze-dried liposomal ciprofloxacin powder for pulmonary inhalation aerosol showed a MMAD of 2.8 μm and FPF of 60.6 per cent, indicative of enhanced lung deposition39.
Mpex Pharmceuticals has been investigating a solution for inhalation formulation of Levofloxacin (Figure 1D; MP-376, Phase III) with PARI eFlow technology40. This formulation is being developed as a treatment of CF, chronic respiratory infections due to P. aeruginosa, other serious bacterial pathogens and COPD with several clinical trials.
Glycopeptides, macrolides, monobactams, polypeptides: vancomycin, azithromycin, aztreonam, colistin
Nektar Therapeutics (currently Novartis) developed a vancomycin (Figure 1G) pulmonary inhalation aerosol formulation by freeze-drying for targeting Gram-positive pneumonia, including methicillin-resistant Staphylococcus aureus (MRSA)31.
Azithromycin (Figure 1I ) has been reported for use in patients with CF41. Hickey et al.42 described that LC® Plus, manufactured by PARI Respiratory Equipment, Inc., had potential as an optimised nebuliser for azithromycin solution with high emitted dose and proportion of fine particles by mass. Zhao et al.43 reported a DPI formulation of azithromycin as an alternative to nebuliser-based formulations. Recently, lyophilised aztreonam (Figure 1J) for inhalation was launched in the US (CAYSTON® 75mg/vial, Gilead Science Inc.), administrated by reconstitution with one millilitre of sterile diluents and using an Altera® nebulise13. De Boer et al.44-45 reported that colistin (Figure 1H) sulphate DPI formulations had potential as an alternative to nebulisation for patients with CF.
Inhalation aerosols for antibiotics in clinical trials
Recently, several clinical trials of pulmonary inhalation aerosol for antibiotics46 have been ongoing worldwide. Some examples are listed in Table 2.
| Drug | Phase | Type | Condition | Company | Location |
| Aztreonam | Approved for marketing | Lyophilized drug for nebulization | Cystic Fibrosis | Gilead Sciences | US|EU |
| Tobramycin | Phase III | Dry powder for inhalation | Cystic Fibrosis | Novartis | US|EU|Mexico|Chile|Canada |
| Levofloxacin (MP-376) | Phase III | Solution for inhalation | Cystic Fibrosis | Mpex Pharmaceuticals | US |
| Amikacin (BAY41-6551) | Phase III | Solution for inhalation | Pneumonia | Bayer | EU|Japan |
| Moxifloxacin (BAY12-8039) | Phase III | Solution for inhalation | Pneumonia, Aspiration, Lung Abscess | Bayer | EU |
| Amikacin (Arikace™) | Phase I | II | Liposomal formulation for nebulization | Cystic Fibrosis | Transave Inc. | US|EU |
| Ciprofloxacin (BAYQ3939) | Phase I | Dry powder for inhalation | Pseudomonas | Bayer | US |
Phase I pharmacokinetic-pharmaco – dynamic (PK-PD) studies are ongoing for formulations of ciprofloxacin (BAY Q3939, Bayer) and amikacin (ArikaceTM, Transave Inc.). ArikaceTM can be delivered with a small droplet size of aerosol to facilitate through nebulisation. A clinical trial is underway for sustainedrelease pulmonary inhalation aerosol of a liposomal formulation.
Phase III clinical studies of tobramycin DPI formulation of Novartis are ongoing to evaluate efficacy, safety and tolerability as an alternative to tobramycin solution for inhalation by nebulisation.
In addition to clinical studies in CF patients with lung infections, recent clinical trials of aerosolised antibiotics have involved non-CF patients that have lung infections such as pneumonia47.
Conclusions
The renewed interest in old antibiotic drugs delivered by inhalation to treat pulmonary infections in a targeted manner has increased significantly in medical practice in recent years; the need and application will continue to increase due to the demonstrated advantages of targeted pulmonary drug delivery by inhaled aerosols and that new antibiotic drug entities are not being discovered as rapidly 48.
Recent research in pulmonary inhalation aerosol delivery of antibiotics is still at the exploratory stage with the exception of some cases. At present, several studies focus on dried powder inhalers (DPIs) for high lung deposition of antibiotics directly to the airways where the infections reside.
Targeted delivery of antibiotics with effective lung deposition drug concentrations to the infection site decreases the risk of systemic side effects which are seen with long-term use of oral or intravenous antibiotic therapy49. Pulmonary inhalation aerosol delivery of antibiotics has been demonstrated to have significant clinical potential in the targeted and effective treatment of pulmonary infections with significantly reduced side effects and reduced formation of drug-resistant bacteria due to enhanced targeting directly to the site of the infection.
References
1. Smaldone GC and Palmer LB. (2000) Aerosolized Antibiotics: Current and Future. Respiratory Care, 45:667-675
2. Flume P and Klepser ME. (2002) The rationale for aerosolized antibiotics. Pharmacotherapy, 22: S71-S79
3. Craig WA. (1997) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1-12
4. Fitzsimmons SC. (1993) The changing epidemiology of cystic fibrosis. J Pediatr 122:1-9
5. Beringer PM.(1999) New approaches to optimizing antimicrobial therapy in patients with cystic fibrosis. Curr Opin Pulm Med 5:371-7
6. Moss RB. (2001) Administration of aerosolized antibiotics in cystic fibrosis patients. Chest 120:107-113
7. Sexauer WP and Fiel SB. (2003) Aerosolized antibiotics in cystic fibrosis. Semin Respir Crit Care Med 24:717-726
8. Gibson RL, Burns JL, Ramsey BW. (2003) Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 168:918-951
9. Mendelman PM, Smith AL, Levy J, Weber A, Ramsey B, Davis RL. (1985) Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am Rev Respir Dis 132:761-7
10. Eisenberg J, Pepe M, Williams-Warren J. (1997) A comparison of peak sputum tobramycin concentration in patients with cystic fibrosis using jet and ultrasonic nebulizer systems. Aerosolized tobramycin study group. Chest 111:955-62
11 Ramsey BW, Burns J, Smith A. (1997) Safety and efficacy of tobramycin solution for inhalation in patients with cystic fibrosis: the results of two phase III placebo controlled clinical trials. Pediatr Pulmonol suppl 14:137-8
12. Novartis pharmaceutical Inc. Tobramycin solution for inhalation (2010) Available from: http://www.tobitime.com
13. Gilead. Aztreonam for inhalation solution (2009) Available from: http://www.gilead.com/pipeline
14. Hickey A.J. and Mansour H.M. (2009) Chapter 5: Delivery of Drugs by the Pulmonary Route. Modern Pharmaceutics Volume 2: Applications and Advances Fifth Edition. Taylor & Francis, Inc., New York. 191-219
15. Beasley R and Hendeles L. (1999) Preservatives in nebulizer solutions: risks without benefit-a further comment. Pharmacotherapy 19:473-4
16. Beasley R, Burgess C, Holt S. (2001) Call for worldwide withdrawal of benzalkonium chloride from nebulizer solutions. J Allergy Clin Immunol 107:222-3
17. Asmus MJ, Sherman J, Hendeles L. (1999) Bronchoconstrictor additives in bronchodilator solutions. J Allergy Clin Immunol 104:553-60
18. De Boer AH, Winter HMI, Lerk CF. (1996) Inhalation characteristics and their effect on in-vitro drug delivery from dry powder inhalers. Int J Pharm 130:231-244
19. Hickey A.J. and Mansour H.M. (2008) Chapter 44: Formulation Challenges of Powders for the Delivery of Small Molecular Weight Molecules as Aerosols. Modified-Release Drug Delivery Technology Second Edition. Informa Healthcare, New York. 573-602
20. Steckel H and Müller BW. (1997) In vitro evaluation of dry powder inhalers. I. Drug deposition of commonly used devices. Int J Pharm 154:19-29
21. Srichana T, Martin GP, Mariott C. (1998) Dry powder inhalers: The influence of device resistance and powder formulation on drug and lactose deposition in vitro. Eur J Pharm Sci 7:73-80
22. Inactive ingredient database for approved drug products by FDA. (2010) Available from: http://www.accessdata.fda.gov/scripts/cder/iig/ index.cfm
23. Pilcer G and Amighi K. (2010) Formulation strategy and use of excipients in pulmonary drug delivery. Int J Pharm 392:1-19
24. Clay MM and Clarke SW. (1987) Wastage of drug from nebulisers: a review. J R Soc Med 80:38-39
25. Newhouse MT, Hirst PH, Duddu SP, et al. (2003) Inhalation of a dry powder tobramycin PulmoSphere formulation in healthy volunteers. Chest 124:360-6
26. Geller DE, Konstan MW, Konstan MW, et al. (2007) Novel tobramycin inhalation powder in cystic fibrosis subjects: pharmacokinetics and safety. Pediatr Pulm 42:307-13
27. Pilcer G, Sebti T, Amighi K. (2006) Formulation and characterization of lipid-coated tobramycin particles for dry powder inhalation. Pharm Res 23:931-40
28. Pilcer G, Goole J, Van Gansbeke B, et al. (2008) Pharmacoscintigraphic and pharmacokinetic evaluation of tobramycin DPI formulations in cystic fibrosis patients. Eur J Pharm Biopharm 68:413-21
29. Parlati C, Buttini F, Ammit AJ, et al. (2009) Pulmonary spray dried powders of tobramycin containing sodium stearate to improve aerosolization efficiency. Pharm Res 26:1084-92
30. Garcia-Contreras L, Fiegel J, Telko MJ, et al. (2007) Inhaled large porous particles of capreomycin for treatment of tuberculosis in a guinea pig model. Antimicrob Agents Chemother 51: 2830-6
31. Nektar. Anti-Infectives. (2009) Available from: http://www.nektar.com/product_pipeline/all_phases.html
32. Shah SP, Misra A. (2004) Liposomal Amikacin Dry Powder Inhaler: Effect of Fines on In Vitro Performance. AAPS PharmSciTech 5: E65
33. Omri A, Beaulac C, Lagacé J, et al. (1994) Pulmonary retention of free and liposome-encapsulated tobramycin after intratracheal administration in uninfected rats and rats infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother 38:1090 -5
34. Mugabe C, Azghani AO, Omri A. (2005) Liposomemediated gentamicin delivery: development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J Antimicrob Chemother 55: 269 -71
35. Adi H, Young PM, Chan HK, et al. (2008) Cospray dried antibiotics for dry powder lung delivery. J Pharm Sci 97:3356-66
36. Adi H, Young PM, Chan HK, et al. (2010) Controlled release antibiotics for dry powder lung delivery. Drug Dev Ind Pharm 36:119-126
37. Tsifansky MD, Yeo Y, Evgenov OV, et al. (2008) Microparticles for inhalational delivery of antipseudomonal antibiotics. AAPS J 10:254-60
38. Zhao H, Liu H, Hu T, et al. (2009) Preparation of microsized spherical aggregates of ultrafine ciprofloxacin particles for dry powder inhalation (DPI). Powder Technol 194:81-6
39. Sweeney L, Wang Z, Finlay W, et al. (2005) Spray-freezedried liposomal ciprofloxacin powder for inhaled aerosol drug delivery. Int J Pharm 305:180-5
40. Waldrep JC and Dhand R. (2008) Advanced nebulizer designs employing vibrating mesh/aperture plate technologies for aerosol generation. Curr Drug Deliv 5:114 -9
41. Prescott WA and Johnson CE. (2005) Antiinflammatory therapies for cystic fibrosis: past, present, and future. Pharmacotherapy 25:555-73 42. Hickey AJ, Lu D, Ashley ED, et al. (2006) Inhaled azithromycin therapy. J Aerosol Med. 19:54-60
43. Zhao M, Yao Y, Ren Y, et al. (2008) Formulation, characteristics and aerosolization performance of azithromycin DPI prepared by spray-drying. Powder Technol 187:214-21
44. De Boer AH, Le Brun PPH, van der Woude HG, et al. (2002) Dry powder inhalation of antibiotics in cystic fibrosis therapy, part 1: development of a powder formulation with colistin sulfate for a special test inhaler with an air classifier as de-agglomeration principle. Eur J Pharm Biopharm 54:17-24
45. Le Brun PPH, de Boer AH, Mannes GPM, et al. (2002) Dry powder inhalation of antibiotics in cystic fibrosis therapy: part 2 Inhalation of a novel colistin dry powder formulation: a feasibility study in healthy volunteers and patients. Eur J Pharm Biopharm 54:25-32
46. Search for clinical trials. 2010. available from: http://www.clinicaltrial.gov/ct2/search
47. Misra A, Jinturkar K, Patel D, et al. (2009) Recent advances in liposomal dry powder formulations: preparation and evaluation. Expert Opin Drug Deliv 6:71-89
48. Traini D and Young PM. (2009) Delivery of antibiotics to the respiratory tract: an update. Expert Opin Drug Deliv 6: 897-905
49. Kuhn RJ. (2001) Formulation of aerosolized therapeutics. Chest 120:94S–98S
About the Authors
Chun-Woong Park received his MS in pharmaceutics at 2005 and PhD in Physical pharmacy at 2008 from Sungkyunkwan University, Republic of Korea. Currently, he is a postdoctoral visiting scholar at University of Kentucky College of Pharmacy, Department of Pharmaceutical science-Drug Development Division. His research focus is on advanced particle engineering design for dry powder inhalation aerosols.
Don Hayes, Jr., MD, MS is an Assistant Professor of Pediatrics, Internal Medicine, and Surgery at the University of Kentucky College of Medicine. He is the Medical Director of the Advanced Lung Disease and Lung Transplant Programs at the University of Kentucky Medical Center. He graduated with honours from the University of Kentucky College of Medicine. He completed a combined Internal Medicine and Paediatrics residency at East Carolina University Brody School of Medicine. He then completed a combined Adult and Paediatric Pulmonary Fellowship at the University of Wisconsin School of Medicine and Public Health. He is a UNOS certified Transplant Pulmonologist. He is an active member of the American Thoracic Society, American College of Chest Physicians, and International Society for Heart and Lung Transplantation.
Dr. Heidi M. Mansour, Ph.D., R.Ph. is Assistant Professor of Pharmaceutics and Pharmaceutical Technology in the Drug Development Division at the University of Kentucky College of Pharmacy and Faculty Associate in the University of Kentucky Center for Membrane Sciences. She currently holds Faculty appointments and Graduate Faculty appointments in the College of Pharmacy at the University of Kentucky and University of North Carolina-Chapel Hill and in the NSF IGERT and NSF REU research training programs joint with the University of Kentucky College of Engineering. Prior to her faculty appointment at the University of Kentucky College of Pharmacy, she was an Instructor, Postdoctoral Fellow and Scholar at the University of North Carolina at Chapel Hill, School of Pharmacy, in the Division of Molecular Pharmaceutics. She earned a BS degree in Pharmacy with Honours & Distinction, PhD in Pharmaceutical Sciences with a PhD Major in Drug Delivery/Pharmaceutics (School of Pharmacy) and a PhD Minor in Advanced Physical & Biophysical Chemistry (Dept of Chemistry), all from the University of Wisconsin-Madison. Dr. Mansour is a member of several professional organisations, including the American Association of Pharmaceutical Scientists (AAPS), European Federation for Pharmaceutical Sciences (EUFEPS), European Cystic Fibrosis Society (ECFS) among others. She serves on the Editorial Advisory Boards (EAB) of several journals, and as a Delegate to the United States Pharmacopeia (USP). She regularly serves as an expert reviewer for 30 scientific journals and several book publishers. She has authored many peer-reviewed scientific publications (including several book chapters and invited papers), in addition to many poster and speaker presentations at national and international conferences. More information can be found on her websites: http://myprofile.cos.com/surfchemlungmed http://pharmacy.mc.uky.edu/faculty/ HeidiMansour.php http://openwetware.org/wiki/User: Dr._Heidi_M._Mansour http://www.research.uky.edu/Membrane/faculty.html http://igert.engr.uky.edu/faculty.html ttp://nsfreu.engr.uky.edu/facultymentors.html email: [email protected]