Extractables – leachables correlations for packaging
Posted: 22 August 2017 | Dr Dennis Jenke | 1 comment
Plastic materials, widely used in pharmaceutical packaging systems, can interact with the packaged product by transferring leachables. Leaching is important, as foreign leachable impurities can adversely affect the quality, efficacy and safe use of the packaged product, due to their chemical or physical nature, reactivity, and/or toxicity.

Although various definitions exist for the term ‘leachables’, they share three common themes:
- Leachables are present in the drug product (and are surfaced, identified and quantified by drug product testing)
- Leachables accumulate in the drug product under its typical conditions of manufacturing, storage, distribution and clinical use (and reasonable accelerations thereof)
- Leachables are derived from the packaging system, including its primary and secondary components. Although a packaged drug product may contain foreign impurities that are not packaging-related (for example, environmental contaminants), such impurities are not leachables.
Ideally, the control and assessment of leachables is enabled by analytical screening of the drug product for packaging-related foreign impurities. Practically, this activity may not be possible or appropriate. For example, drug products may be so chemically complex that they cannot successfully be screened for leachables, whose identities and concentrations are unknown until established, with the required specificity and sensitivity. Additionally, drug product testing does not effectively control leachables, as so doing represents a reactive approach that is poorly aligned with Quality by Design principles.
To deal with such situations, the concept of extractables was developed. An extractable is “a chemical entity, organic or inorganic, that is released from a test article (which could be a packaging system, one of its components, or one of its materials of construction) during controlled extraction studies performed under laboratory conditions”. By convention extractables and leachables are related to some extent.
Best practice recommendations and compendial standards for extractables and leachables note that a leachables-extractables correlation (either qualitative or quantitative) should be established by linking actual drug product leachables with extractables from corresponding controlled extraction studies performed on either the packaging system itself, individual components, or individual materials of construction. These same recommendation and standards note that, “leachables-extractables correlations are important for several reasons, including justifying the use of routine extractables release tests of packaging components as an alternative to leachables testing during stability studies for high-risk drug products, establishing the source of a leachable producing an OOS result for a low-risk drug product, change control, and ongoing quality control, etc”.
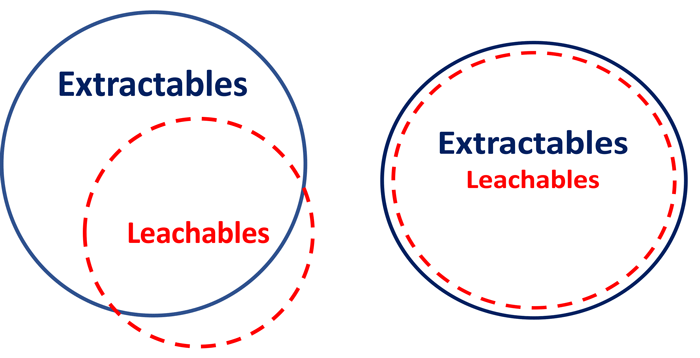

Figure 1. The relationship between extractables and leachables. (Left )(A) Using the ‘old’ definitions, this diagram expresses the situation where ‘typically leachables are a subset of extractables’. The relationship between extractables and leachables is actually situational and can vary from a full and complete correlation to a poor and largely incomplete correlation. It is rare that extractables and leachables are completely uncorrelated, either directly or by extension. (B) Using the proposed definitions, extractables and leachables will be highly correlated, with extractables representing worst-case leachables, by both definition and intent.
Acceptance, adoption and implementation of the recommendations for performing extractables-leachables correlations have led to inevitable but unfortunate generalisations. Instead of fostering understanding and harmonisation, generalisations such as “leachables are typically a sub-set of extractables”, either verbalised or characterised as shown in Figure 1, have created confusion and discord among practitioners of the art and science of extractables and leachables assessment.
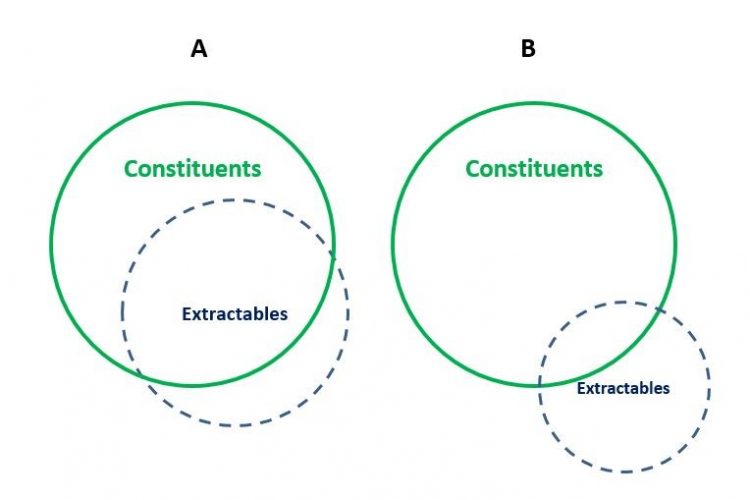

Figure 2. The relationship between constituents and extractables using the proposed definitions. (A) Extraction conditions are more ‘aggressive’ (stronger solvent, higher temperature, longer duration) and thus constituents are solubilised to a greater extent. (B) Extraction conditions are less ‘aggressive’ (weaker solvent, lower temperature, shorter duration) and thus constituents are solubilised to a lesser extent. Those extractables that are not constituents include processing residuals, degradation products of the constituents, and environmental contaminants.
Driving consistency and understanding
The root of this confusion and discord is no more complex than the observation that the terms extractables and leachables, as defined previously, are not concise. For example, since the definition of an extractable does not specify a specific controlled extraction study, one intuitively understands that an extractables profile (a list of extractables and their concentrations) will differ, depending on the purpose for which the profile will be used, which drives the experimental conditions of the controlled extraction study. That is, an extractables profile obtained using ‘aggressive’ extraction conditions is likely to be different from an extractables profile generated using more ‘gentle’ conditions.
Moreover, since the definition of a leachable does not specify a specific drug product or a specific set of clinical circumstances, one understands that chemically different drug products packaged in the same packaging system could have different leachables profiles, and that even a single drug product in a specified packaging system could have a different leachables profile depending on the clinical circumstances (for example, beginning and end of shelf life). Thus, it is clear why a generalisation such as that shown in Figure 1 leads to discord and confusion, since the relationship between extractables and leachables differs depending on the circumstances.
Some of the confusion and discord can be managed if the terminology is tightened up, specifically as it relates to extractables. Consider the purposes for which a controlled extraction study might be performed:
- Establishing the complete composition of a test article (for example, to facilitate material selection)
- Establishing the level of one or more targeted substances in a test article (for example, quality control of incoming raw materials, change control)
- Establishing the levels of leachables in a drug product (for example, as the basis of a toxicological safety risk assessment).
It is intuitive that extractions designed for these very different purposes could be quite different in their experimental conditions and would produce compositionally different extracts containing different sets of extracted substances. If one applies the term extractables to substances present in any or all of these extracts, then one clearly has created a situation where extractables-leachables correlations can be complex and confusing. However, if one adopts a terminology where:
- The term ‘constituents’ is applied to those substances present in an extract generated to establish the composition of a test article
- The term ‘extractables’ is applied to those substances present in an extract generated to mimic the leaching characteristics of a drug product
- The term ‘control target’ is applied to those substances present in an extract generated to control the composition of incoming materials.
Then one has created a situation in which extractables – leachables correlations are simpler, clearer, more useful and direct.
In essence, one has taken a universe which had two related populations – extractables and leachables – and changed that universe to one that contains four, more closely-related populations: constituents, extractables, control targets and leachables. The question now becomes: “how are these populations related?”
If any of these stipulations are not met, then obtaining an excellent correlation is less likely, with the degree of divergence between extractables and leachables reflecting the degree to which the experimental circumstances deviate from the clinical circumstances.
In this context, an ‘excellent’ correlation means that each leachable in the drug product is also an extractable in the extract, and the concentration of the extractable in the extracts is equal to or slightly greater than the leachable’s concentration in the drug product (worst-case).
Although the more focused definition of extractables will shrink the extractables-leachables gap, it will not close it in all circumstances. For example, an extraction study designed to forecast leachables may do so for a group of drug substances spanning a range in composition. While the resulting extractables profile will be a worst-case for all drug products in the group, it is possible that the profile would be exaggerated for any one drug product in the group, leading to a poorer extractables-leachables correlation for that specific product.
Correlating constituents with extractables and leachables
In general, an ‘aggressive’ extraction is required to establish the composition of a plastic material, component or packaging system, including a list of all the constituents and their levels; for example, dissolution, exhaustive extraction or exaggerated extraction. Relatively speaking, interactions between a drug product and its packaging system are much less aggressive than the composition-focused extractions, and herein lies the root of the disconnect between constituents and extractables.
The more aggressive the interaction between a drug product and its packaging, the more aggressive will be the extraction conditions employed in an extractables study, the greater number and quantity of constituents that will be present in the extracts as extractables, and the better will be the constituents-extractables correlation Figure 2. The less aggressive the interaction between a drug product and its packaging, the less aggressive will be the extraction conditions employed in an extractables study, the fewer number and lower quantity of constituents that will be present in the extracts as extractables, and the poorer the constituents-extractables correlation.
Extractables could include substances that are not constituents, such as products that arise from the processing of a packaging system, including processing residuals (eg, bonding solvents), degradation products of the constituents (eg, oxidized forms of antioxidants), and environmental contaminants (for example, DEHP in non-DEHP materials).
As leachables are highly correlated with extractables, the relationship between constituents and leachables is largely the same as the relationship between constituents and extractables.
Correlating control targets
A control target is measured in an extract for comparison. In quality control, the measured level of the control target is compared to a specification to establish an incoming article’s suitability for use. In change control, the measured level of the control target in a current article is compared to the level in a changed article to establish the effect of the change. In either circumstance, the ultimate purpose of the exercise is to control the level of certain leachables in the drug product and thus the control target is chosen to target the point of control. In the event of adequate constituent-extractable-leachable correlation, then the control target will be a constituent. If the correlation is poor, then the control target is likely an extractable. If the substance to be controlled is not related to composition (e.g., processing residual, environmental contaminant), then the control target is an extractable.
Conclusion
Limiting the term extractables to extraction studies for forecasting leachables and introducing the terms constituent to address extraction studies for establishing an item’s composition, and control target to address extraction studies for exercising control of either the supply chain or change control, leads to more concise, consistent and useful correlations between leachables and these packaging-related entities. These improved correlations facilitate the qualification, control and life-cycle management of packaging systems and packaged drug products.
BIOGRAPHY
Dr Dennis Jenke is Chief Executive Scientist at Triad Scientific Solutions. He is the author of Compatibility of Pharmaceutical Solutions and Contact Materials, Safety Considerations Associated with Extractables and Leachables, and a contributing author to the Leachables and Extractables Handbook. Dr. Jenke is a member of numerous industry groups whose charter is to establish best demonstrated practices in the area of material/solution compatibility. Dr Jenke can be contacted at [email protected].
References
- Safety Thresholds and Best Practices for Extractables and Leachables in Orally Inhaled and Nasal Drug Products. PQRI Leachables and Extractables Working Group. September 9, 2006. Available at: pqri.org/pdfs/LE-Recommendations-to-FDA-09-29-06.pdf.
- 〈1〉 PLASTIC MATERIALS OF CONSTRUCTION. USP39-NF34, page 493. Official May 1, 2016.










Very interesting article, thanks for sharing such a valuable information.