Cellular reprogramming and its implications for therapeutic applications
Posted: 29 May 2009 | Dr Jens Schwamborn, Groupleader, Münster University and Lai Wen, Visiting Scholar, Münster University | No comments yet
Nearly fifty years ago, it was hypothesised that terminally differentiated cells such as fibroblasts could be forced to take on a pluripotent state, similar to the embryonic stem cells (ES cells). The basis of the concept is the observation that all cell types, with minor exceptions, have the same genetic code. The only difference is how the code is read. This ability of differentiated cells to acquire a pluripotent state or, more generally, the process of cell fate conversion is termed cellular reprogramming.
Nearly fifty years ago, it was hypothesised that terminally differentiated cells such as fibroblasts could be forced to take on a pluripotent state, similar to the embryonic stem cells (ES cells). The basis of the concept is the observation that all cell types, with minor exceptions, have the same genetic code. The only difference is how the code is read. This ability of differentiated cells to acquire a pluripotent state or, more generally, the process of cell fate conversion is termed cellular reprogramming.
Nearly fifty years ago, it was hypothesised that terminally differentiated cells such as fibroblasts could be forced to take on a pluripotent state, similar to the embryonic stem cells (ES cells). The basis of the concept is the observation that all cell types, with minor exceptions, have the same genetic code. The only difference is how the code is read. This ability of differentiated cells to acquire a pluripotent state or, more generally, the process of cell fate conversion is termed cellular reprogramming. Reprogramming here means transition between cell fates or dedifferentiation. The classic and earliest change of direction of cell differentiation comes from the research of nuclear transfer. Another milestone showing that the nuclear transfer technology can reverse the cell fate of somatic cells to pluripotent stem cells was the production of the normal adult sheep Dolly. Recent and spectacular advances in this field fell in 2006 when mouse fibroblasts were reprogrammed to induced pluripotent stem (iPS) cells. The next big step was the in vivo reprogramming of adult pancreatic exocrine cells to beta cells. This series of excellent work turns back the clock of somatic cells to create the first (iPS) cells, or stem cells, made without the use of embryos. In this article, we will focus on cellular reprogramming; in particular on transcription factor induced reprogramming as well as its implications for therapeutic use.
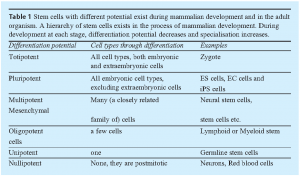
Hierarchy of Stem Cells
Stem cells are unspecialised cells that are defined by at least two characteristics: they have the ability to divide for indefinite periods (self-renewal) and they have the potential to differentiate to specialised cells (potency). During the development of multicellular organisms or by giving the right signals in vitro, stem cells can give rise to the many different cell types that make up the organism. This means that stem cells have the potential to develop into mature cells like heart cells, skin cells, or nerve cells.
Based on the differentiation potential, stem cells are defined as totipotent, pluripotent, multipotent or unipotent stem cells, and eventually nullipotent cells. An example for totipotency is a fertilised egg that can develop to an adult organism consisting of more than 200 cell types. Pluripotent stem cells such as ES cells originating from the inner cell mass can differentiate into all three germ layers except the ectoderm, forming any tissue in the body. Multipotent stem cells exist in the adult organism as tissue specific stem cells. They can give rise to a number of cells, but only to those belonging to a closely related family of cells or confined to a specific tissue.
Among those, because of their potential to differentiate to all three germ layers and unlimited life span in culture, the pluripotent stem cells are of particular interest for science. Furthermore, pluripotent stem cells can be genetically manipulated and could be potentially utilised as a source for cell replacement approaches for restoring tissue function, providing promise for therapeutic applications in cure of many complicated human diseases, such as Parkinson`s disease, diabetes and many more. Research on ES cells holds huge promise for understanding and treating those diseases. However, because of religious and ethical reasons the therapeutic use of embryo derived stem cells is not free of criticism. Fortunately recently developed methods may bypass some of these objections.
Reprogramming of somatic cells into iPS Cells
For a long time it was believed that the differentiation from the fertilised egg to specialised cells and eventually an adult organism is an irreversible one-way process. However, this belief was overthrown by a series of discoveries in the past several decades, first by nuclear transfer and, more recently, by the production of iPS cells. These experimental manipulations have shown that terminally differentiated cells can be reprogrammed into stem cells.
In 2006, Shinya Yamanaka and colleagues used retroviruses to ectopically express the four transcription factors Oct4, Sox2, c-Myc and Klf4 in mouse fibroblasts. Expression of these four factors was enough to reprogram the fibroblast into pluripotent stem cells1. These cells are called induced pluripotent stem (iPS) cells. When tested across a rigorous set of assays, encompassing morphological, molecular, and functional attributes these iPS cells are almost identical to ES cells2-4. They express mouse ES-cell markers, have similar gene expression profiles, form teratomas when injected into nude host and contribute to germline in chimera. One year later, direct reprogramming of iPS cells from human fibroblasts was achieved by introducing the same four factors Oct4, Sox2, c-Myc and Klf4 or Nanog and Lin285,6. Obviously these iPS cells hold great promise for the study and therapy of human diseases7,8. However, a key question raised by transcription factor-induced reprogramming is how the four factors act to bring about this change.
Although we can basically attribute the transition of cell fate to epigenetic change in cells, the molecular mechanisms underlying the reprogramming of iPS cells have not yet been fully characterised. The complete reprogramming takes 20 to 30 days, but it was demonstrated that exogenous factors are required for only about 10 days, after which cells enter a self-sustaining pluripotent state9. Therefore, it seems that reprogramming is a gradual process including sequential change of epigenetic state and sequential activation of pluripotency-associated genes10,11,12.
Before reprogramming by ectopic expression of transcription factors, somatic nuclear transfer is the earliest exploration to reprogram differentiated cells to stem cells13,14. Other strategies include cell fusion, cell extracts or culture induced reprogramming15-17. The differences among cell fusion, nuclear transfer and reprogramming by ectopic expression of transcription factors might be mostly technical, while they may share the same underlying mechanism of reprogramming: from nuclear reprogramming to cellular reprogramming. First, an exogenous factor may be imposed to the target cell. This can be a new cytoplasm (nuclear transfer), a whole cell (cell fusion) or several transcription factors (transcription factor induced reprogramming). Second, these mentioned exogenous factors can all be summarised as new transcriptional regulators. It is very important for the nuclear chromatin to be exposed to a new environment, resulting in changes in gene expression and eventually the whole epigenetic status changes. Finally, after the expression profile changed, the phenotype of the cell is reprogrammed.

Advanced reprogramming
As described iPS cells were first reprogrammed from fetal and adult fibroblasts to an embryonic-like state with four factors. After that, a lot of work has been done to optimise this reprogramming. Furthermore, a multitude of cell types have been reprogrammed, encompassing cells originating from all three germ layers. These include stomach cells18, liver cells18,19, pancreatic beta cells20, lymphocytes21, neural progenitor cells22,23 and keratinocytes24,25.
Additional experiments showed that the oncogene c-Myc is dispensable for direct reprogramming of mouse fibroblasts26,27. Because neural stem cells (NSCs) endogenously express Sox2 and c-Myc, both factors are dispensable for reprogramming of NSCs into iPS cells22. More recently, iPS cells have finally been produced from neural stem cells by only one factor, Oct423. These results suggest that endogenous expression could have a role in the reprogramming process and that reprogramming is mainly a product of two variables, the starting cell type (endogenous) and the reprogramming factors (exogenous).
Considering that the ectopic expression of c-Myc can induce tumour development and that retroviruses themselves can cause insertional mutagenesis, the generation of iPS cells with a minimal number of factors may hasten the clinical application of this approach. Cell types that express one or more of the reprogramming factors are easier to reprogram into iPS cells than cells with low or null expression of the factors and thus may require less genetic manipulation. Such cell types, including NSCs, may be an appropriate source of cells for attempts to replace viral vector gene delivery systems with transient delivery systems or small compounds to produce iPS cells without genetic manipulation.
Although the efficiency is low, iPS cells without viral integration have been produced by using non-integrating adenoviruses, and even more strikingly by transient transfection of plasmids for the four magic factors Oct4, Sox2, Klf4, and c-Myc9,28. Other reports demonstrated the use of fusion proteins to produce iPS cells29,34 and the application of small molecules or chemicals to improve the efficiency of iPS cell production22.
To generate the ultimate modification-free pluripotent stem cell, an increasing amount of work is dedicated to find a more suitable starting cell type for reprogramming. Interestingly a pluripotent stem cell line has been derived from spematogonial cells of the adult human testis just by growing them in a defined medium30. Obviously this method is a direct and safe access to cell replacement therapy without virally expressed transcription factors and immunological problems.
Another approach that probably has broad medical implications comes from Douglas A. Melton and colleagues31. They used adenoviral transduction to deliver the three factors Pdx1, Ngn3, and MafA to reprogram adult pancreatic exocrine cells into beta-cells. The very particular thing about this approach is that they managed to induce this reprogramming in vivo in an adult mouse.
Outlook and potential therapeutic applications
The current advances in reprogramming of somatic cells into ES-cell like iPS cells and the direct conversion of cell types suggest that reprogramming depends, to a large extent, on over-expression of particular genes (key transcription factors) followed by induction of overall epigenetic network changes. Therefore it seems that the choice of the “right” reprogramming factors is the most important thing during the whole approach. Although regulatory networks play an important role, there is the longstanding concept of “master genes” that determine cell fate. For example, Oct4 is generally considered to be the master gene for the maintenance of the pluripotent state of ES cells.
Although the cocktail of factors to induce iPS cells varies, Oct4 never can be omitted. Another example is MyoD, the master gene for muscle differentiation, which is sufficient to reprogram non-muscle cell types into muscle32,33. Therefore, in the near future, one can expect that more and more master transcription factors for certain cell types will be described.
The production of iPS cells is in the centre of stem cell research and regenerative medicine. These studies have at least three implications:
- The most striking revolution may take place in the cell replacement therapy. iPS cells can be produced from an individual patient. Therefore, if these cells are used for replacement approaches there will be no problem with the immune rejection. Meanwhile it circumvents the longstanding heated debate concerning the moral and ethical use of ES cells from embryos. For cell replacement therapy, it should be noted that teratoma formation is an inherent feature of ES cells and iPS cells. As this tumorigenic activity is lost following differentiation, however, differentiated cells are expected to be safe for therapeutic use (provided that undifferentiated cells are removed). Because it is obviously critical to control the differentiation precisely, in the future it will be important to find out how to differentiate iPS cells in all the cell types that are needed for replacement therapies. Furthermore the production of virus-free iPS cells is a prerequisite for the therapeutic use of these cells.
- Daley and colleagues8 reported the production of human iPS cell lines for ten diseases, ranging from disorders, like adenosine deaminase deficiency to complex conditions, like Parkinson’s disease and Type 1 diabetes. Such disease-specific stem cells offer an unprecedented opportunity to recapitulate both normal and pathologic human tissue formation in vitro, thereby enabling disease investigation and drug development. Patient-specific iPS cells also have the potential to speed up drug discovery and to provide valuable tools for toxicology testing. Development in this field will truly forward one step closer to the so called personalised medicine.
- Finally iPS cells have great potential as a research tool. Firstly, because iPS cells maintain the ability to undergo differentiation to all cell lineages, they can provide a universal cell source for the study of the cell biology of embryogenesis. Secondly, how the reprogramming occurs exactly remains largely unknown. However, to understand this process in detail is critical to understand how cell fate is determined.
The first examples how iPS cells could be used to treat diseases in mice are already available. Mice with humanised sickle cell anemia can be cured by transplantation with hematopoietic progenitors that have been generated in vitro from autologous iPS cells following correction of the defective gene by homologous recombination21.
Although the production of iPS cells is indeed an incredible step forward in stem-cell research, it is in fact just the beginning of a long road.
References
- Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663-676 (2006).
- Maherali, N., et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1, 55-70 (2007).
- Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313-317 (2007).
- Wernig, M., et al. In vitro reprogramming of fibroblasts into a pluripotent ES -cell-like state. Nature 448, 318-324 (2007).
- Takahashi, K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861-872 (2007).
- Yu, J., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, N.Y 318, 1917-1920 (2007).
- Dimos, J.T., et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science (New York, N.Y 321, 1218-1221 (2008).
- Park, I.H., et al. Disease-specific induced pluripotent stem cells. Cell 134, 877-886 (2008).
- Stadtfeld, M., Maherali, N., Breault, D.T. & Hochedlinger, K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2, 230-240 (2008).
- Brambrink, T., et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2, 151-159 (2008).
- Yamanaka, S. Strategies and new developments in the generation of patient -specific pluripotent stem cells. Cell Stem Cell 1, 39-49 (2007).
- Jaenisch, R. & Young, R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132, 567-582 (2008).
- Briggs, R. & King, T.J. Transplantation of Living Nuclei From Blastula Cells into Enucleated Frogs’ Eggs. Proceedings of the National Academy of Sciences of the United States of America 38, 455-463 (1952).
- Byrne, J.A., et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 450, 497-502 (2007).
- Pomerantz, J. & Blau, H.M. Nuclear reprogramming: a key to stem cell function in regenerative medicine. Nat Cell Biol 6, 810-816 (2004).
- Harris, H. Hybrid cells from mouse and man: a study in genetic regulation. Arzneimittelforschung 17, 1438-1439 (1967).
- Han, D.W., et al. Pluripotential reprogramming of the somatic genome in hybrid cells occurs with the first cell cycle. Stem Cells 26, 445-454 (2008).
- Aoi, T., et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science (New York, N.Y 321, 699-702 (2008).
- Stadtfeld, M., Nagaya, M., Utikal, J., Weir, G. & Hochedlinger, K. Induced pluripotent stem cells generated without viral integration. Science (New York, N.Y 322, 945-949 (2008).
- Stadtfeld, M., Brennand, K. & Hochedlinger, K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr Biol 18, 890-894 (2008).
- Hanna, J., et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science (New York, N.Y 318, 1920-1923 (2007).
- Shi, Y., et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 3, 568-574 (2008).
- Kim, J.B., et al. Oct4-induced pluripotency in adult neural stem cells. Cell 136, 411-419 (2009).
- Aasen, T., et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol 26, 1276-1284 (2008).
- Maherali, N., et al. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell 3, 340-345 (2008).
- Nakagawa, M., et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26, 101-106 (2008).
- Wernig, M., Meissner, A., Cassady, J.P. & Jaenisch, R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2, 10-12 (2008).
- Okita, K., Nakagawa, M., Hyenjong, H., Ichisaka, T. & Yamanaka, S. Generation of mouse induced pluripotent stem cells without viral vectors. Science (New York, N.Y 322, 949-953 (2008).
- Maherali, N. & Hochedlinger, K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell 3, 595-605 (2008).
- Conrad, S., et al. Generation of pluripotent stem cells from adult human testis. Nature 456, 344-349 (2008).
- Zhou, Q., Brown, J., Kanarek, A., Rajagopal, J. & Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455, 627-632 (2008).
- Van Antwerp, M.E., Chen, D.G., Chang, C. & Prochownik, E.V. A point mutation in the MyoD basic domain imparts c-Myc-like properties. Proceedings of the National Academy of Sciences of the United States of America 89, 9010-9014 (1992).
- Kirillova, I., Gussoni, E., Goldhamer, D.J. & Yablonka-Reuveni, Z. Myogenic reprogramming of retina-derived cells following their spontaneous fusion with myotubes. Developmental biology 311, 449-463 (2007).
- Zhou et al., Generation of Induced Pluripotent Stem Cells Using Recombinant Proteins, Cell Stem Cell (2009), doi:10.1016/j.stem.2009.04.005





