MHRA approves needle-free allergy drug alternative
Posted: 21 July 2025 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
The MHRA’s approval provides eligible patients with a novel delivery method that is convenient and non-invasive.
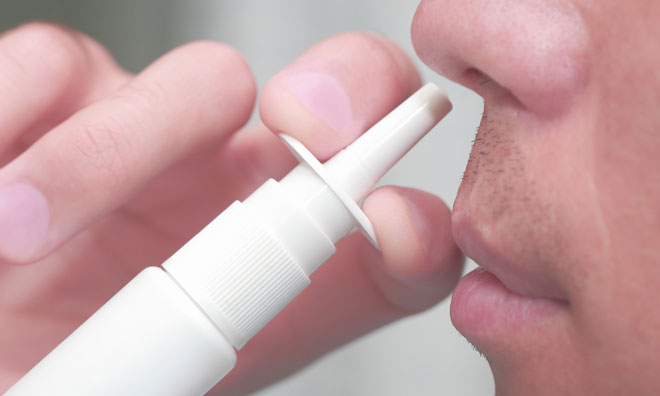

The UK Medicines and Healthcare products Regulatory Agency (MHRA) has approved the first needle-free adrenaline nasal spray for emergency use during allergic reactions (anaphylaxis) in the UK. EURneffy (adrenaline nasal spray) ® is a ready-to-use treatment indicated for adults and children weighing greater than 30kg.
This news follows launch of the medicine in Germany last month.
Clinical data has demonstrated that EURneffy is well absorbed through the nose, providing a simple-to-use alternative. Its other benefits include a 30-month shelf life and no specialist storage is required.
Significance of the MHRA’s approval of EURneffy
“The innovative design of EURneffy with its small size, temperature stability up to 50°C and longer shelf life compared to auto-injectors, encourages patients and caregivers to always carry and use epinephrine at the first sign of symptoms related to a food or venom allergy reaction. We are pleased to receive this approval and expand availability of our epinephrine nasal spray as part of our partnership with ALK-Abelló A/S,” shared Richard Lowenthal, Co-founder, President and CEO of ARS Pharmaceuticals.
“EURneffy [has an innovative design:]… small size, temperature stability up to 50°C and longer shelf life compared to auto-injectors”
The MHRA’s decision about EURneffy provides patients with a nasal adrenaline spray that “addresses several critical barriers seen with the current standard of care, such as portability, fear, hesitancy to act and incorrect administration,” noted Dr Helen Evans-Howells, GP, Allergy specialist and Chair of the Anaphylaxis UK Clinical and Scientific Panel.
This latest authorisation of EURneffy is significant, since prior to approval of EURneffy “there were no regulatory path for non-needle delivery methods [to treat severe allergic reactions]. We worked collaboratively with the US and European agencies to develop the regulatory pathway,” explained Dr Sarina Tanimoto, MBA, Co-Founder and Chief Medical Officer of ARS Pharmaceuticals, in an EPR article last year.
Related topics
Drug Delivery Systems, Drug Development, Drug Markets, Drug Safety, Industry Insight, Regulation & Legislation, Research & Development (R&D), Therapeutics
Related organisations
ARS Pharmaceuticals, Medicine and Healthcare products Regulatory Agency (MHRA)