How & why to build a QbD process to optimise the efficiency of your freeze drying projects
Posted: 1 July 2016 | Biopharma Group | No comments yet
Quality by design (QbD) for lyophilisation is about building a robust process that proactively flags critical points and ensures consistent delivery of the best quality product, not only by minimising risk but also through greater understanding of the process itself…


QbD in Freeze Drying
Quality by design (QbD) for lyophilisation is about building a robust process that proactively flags critical points and ensures consistent delivery of the best quality product, not only by minimising risk but also through greater understanding of the process itself. Regulatory bodies such as the FDA require details of the lyophilisation cycle for product registration but a well-designed cycle also has economic benefits since the best quality cycles are the most efficient in terms of energy usage, time and other resources.
QbD terminology and what it means for freeze dried products
For the final product, a (Quality) Target Product Profile (or (Q)TPP) will need to be established. This will typically define the desired characteristics of the product itself. For generic products, such a profile may be relatively straightforward to define, but for new products, this might not be so simple, as there is less Prior Knowledge (another QbD term) about the product at the early stages of R&D. One of the benefits of the QbD approach is that it is not attempting to robotise the process; in fact, it places significant value on Prior Knowledge, which is recognised as an important factor in reducing risk for the product – one of the core aims of the QbD initiative.
The (Q)TPP for a product might include quantitative limits or ranges for parameters such as moisture content or activity / potency, as well as descriptions or basic categorisation of more qualitative phenomena such as appearance, colour, shape and homogeneity.
Once a (Q)TPP has been established, the “Critical Quality Attributes (CQAs)” for the product can be defined. Not every attribute is necessarily critical to the quality of the product, and so some work will need to be done in order to establish what is critical and what is not. Again, for a generic product, this can prove to be quite a simple process, since much information will exist – some of which can be in the public domain – about the originator’s material. However, for a new product, the process of identifying the CQAs can be much more involved. One approach is to assign a rank order of importance of the various attributes, which can help focus the R&D process onto the most critical parameters and consequently de-risk the product with respect to these parameters. This approach works in much the same way as the “Theory of Constraints” model, where parameters that are initially deemed to be a low risk (or minor constraint) are made subordinate to those that pose a higher risk (or constraint); a solution is provided to deal with the higher risks, which should result in the constraint being lifted or minimised; however, if the constraint is then transferred somewhere else in the system, there may be an element of reiteration required.
For freeze-drying, this might for example involve establishing the liquid state stability of a formulation and understanding its sensitivity to cooling rate or freezing temperature up front, which will hopefully de-risk the filling, loading and freezing process. Focus can then be given to de-risking the primary drying part of the process, which is arguably the highest risk (or constraint) part of the lyophilisation process for many products, and certainly the longest step in a freeze-drying cycle. When it comes to primary drying, much can be done before carrying out practical freeze-drying cycles – in fact, many well-established mathematical equations for heat and mass transfer can be used to establish a possible range of input variables before setting foot in the laboratory. When combined with Prior Knowledge – particularly regarding the risks from the formulation and the equipment, as briefly outlined in the sections below – mathematical modelling can be a powerful tool in one’s Lyo-QbD armoury to mitigate risks.
Risk mitigation: Selecting excipients for the formulation
The choice and balance of excipients in a formulation has a significant bearing on its freeze drying process parameters. Various additives offer protection for different processing stresses, and formulation development aims to create a balance that results in the best possible final product. One challenge is in knowing whether the risks to the product may be better limited by keeping one’s options open with regard to the formulation while carrying out freeze-drying process R&D, or to carrying out formulation development as a separate exercise. Certainly, it has been demonstrated that essential parameters of the active ingredient(s) be investigated prior to starting freeze-drying cycle development, such as solubility in the chosen solvent(s), pH-stability profile, and any chemical incompatibilities identified.
Risk mitigation: Equipment
The freeze drying process itself – and therefore the risk to the product and the robustness of the entire process – is significantly affected by aspects of the specific equipment used, such as shelf cooling rate and the trapping rate limit of the condenser (sometimes known as the ‘choked flow’ point). If the limitations of the equipment are known, this will help ensure smooth scale-up of the process. Specifically, the challenge is in ensuring that whatever process is developed at the laboratory scale, it can be replicated successfully at the pilot- and production- scales. Often the difficulty in this regard comes from the fact that the details of the manufacturing equipment is not necessarily known during the early stages of product R&D – this is almost always the case for a small company developing a promising product, where usually the freeze-drying will be contracted out to a third party – a Contract Research Organisation (CRO) for the R&D and ultimately a Contract Manufacturing Organisation (CMO) once the product reaches the Clinical Trials stage. If the CMO has already been selected early in the R&D process, then the limitations and tolerances of its freeze-dryer(s) can be incorporated into the process, but if this is an unknown during R&D, then additional robustness will need to be built into the freeze-drying cycle in order to make it as “universally applicable” as possible.
Risk mitigation: Critical temperatures
Scientific methods of determining the critical temperatures of a formulation make it possible to design a high quality cycle. The key technologies are freeze drying microscopy (FDM), and thermo-analytical methods such as differential thermal analysis (DTA), electrical impedance analysis (Zsinϕ) and differential scanning calorimetry (DSC). Observing first hand at what temperatures a product exhibits changes in its visible appearance and structure when subjected to the lyophilisation process using freeze-drying microscopy builds confidence for the scientists developing the formulation and the freeze-drying cycle for a product. Thermal and impedance analysis provides data to complement FDM and allows for a greater understanding of how individual components of a formulation – or different phases of the frozen structure – may be behaving, which can often be related to product-related issues such as microcollapse, stability and reconstitution characteristics.
Building a design space: putting it all together
A design space is a multidimensional combination and interaction of input variables and process parameters that have been demonstrated to provide assurance of product quality. A successful and detailed study into all the product and process variables makes it possible to establish a design space that provides repeatable, consistent and provable results. One of the challenges with the design space is that it might need to be more than 3-dimensional, which means it cannot be visualised. In practical reality, it is possible to divide the process into discrete steps, with each step being assessed in terms of its risk, which would give several smaller design spaces that might then be 2- or 3- dimensional.
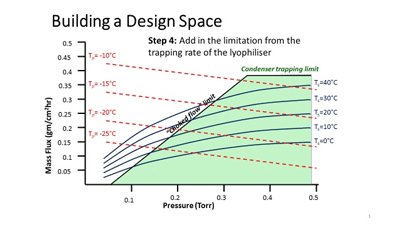

Figure 1: An example of how the design space might be constructed for the primary drying conditions for a freeze-dried product
Figure 1 gives an example of what the boundaries to the Acceptable Space (which is the area within the Design Space deemed to yield a product with the requisite CQAs) might be for the Primary Drying part of the lyophilisation process (adapted after Mockus et al, 2011).
It can be seen that the boundaries of the Acceptable Space are defined by the limitations of the product (critical temperature), of the equipment (process condenser trapping rate, choked flow point) and of process efficiency (in this case, the mass flux through the duct between the chamber and condenser). There may also be additional factors that might be superimposed on top of this diagram, or added as a further dimension in space to give a 3-dimensional ‘box’. One example is to use product resistance (as it increases with time) as the third axis, as it is well established that this can be a significant constraint – especially later in the sublimation process.
While a QbD approach can add significant robustness to a product, and while many of the aspects of the design box can be modelled mathematically (as well as tested by real laboratory studies), it still remains, in part, an iterative process, and it is unlikely that this approach will ever completely negate the requirement for testing in reality. A further consideration with lyophilisation, is that the risks change throughout the process – for example, in the early stages of sublimation, the trapping rate of the process condenser might be the limiting factor to processing efficiency, while later in the same stage of the process, the product itself (specifically the dry layer that sits above the sublimation front) is most likely to be the largest source of impedance to drying.
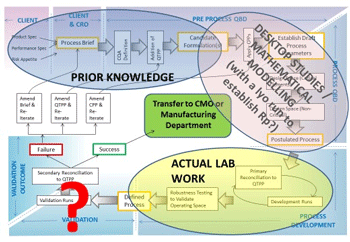

Figure 2: QbD “roadmap” showing possible stages of desktop study and practical work
So, how might the QbD approach be integrated into freeze-drying formulation and process development in practical terms? Figure 2 shows an example of a possible “QbD roadmap” for freeze-dried pharmaceutical products. Starting at the top left of the roadmap, under the principle of ‘Prior Knowledge’, one can set the desired parameters/ outputs. Once these parameters are in place, work clockwise through the flow chart using a combination of candidate formulation assessment, mathematical modelling, and real-life freeze drying (lab work), to ‘design in’ quality at each stage of a product’s design and development, whereupon if the robustness of the process with regard to the various risks is acceptable to the customer/ end user, then it can be considered a success. Perhaps significantly, it can be seen that much of the roadmap involves desktop studies including an assimilation of Prior Knowledge, before practical work actually needs to take place, although it can be useful to gain an understanding of the product resistance (Rp) as it changes throughout the sublimation stage of the cycle, so that this might be built into the Design Space. With a “conventional” 2D space, one would tend to assume a high Rp value throughout the entire process, which may sacrifice efficiency in favour of risk mitigation; a 3D space that takes into account the changing risk might provide a solution to this issue, thus yielding a safe, efficient and robust process.
Reference: L. Mockus et al (2011) Quality by design in formulation and process development for a freeze-dried small molecule parenteral product: a case study, Pharm. Dev. Tech. 16(6) 549-576





