Immune receives FDA guidance for acute myeloid leukaemia Phase III trial
Posted: 28 October 2016 | | No comments yet
The proposed phase III study design reviewed by the FDA guidance focuses on overall survival as the primary endpoint, including Leukaemia Free Survival…
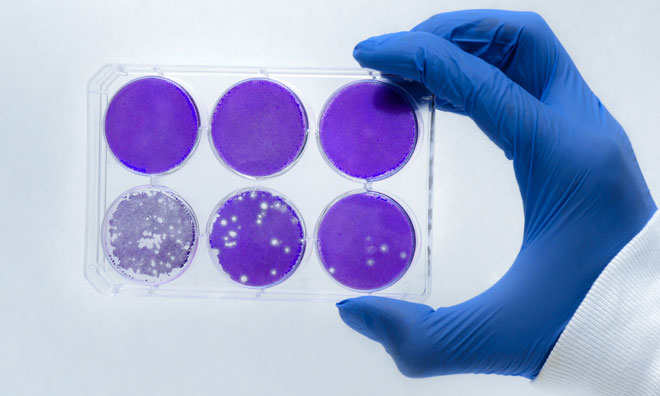

Immune has received guidance from the United States Food and Drug Administration (FDA) on a phase III study for Ceplene in combination with low dose IL-2 for the maintenance of remission in patients with Acute Myeloid Leukaemia (AML).

Ceplene/IL-2 has previously been approved in Europe and Israel following a successful phase III study with Leukemia Free Survival as the primary endpoint. The proposed phase III study design reviewed by the FDA focuses on overall survival as the primary endpoint, along with key secondary endpoints, including Leukaemia Free Survival.
The FDA also provided feedback relating to specific design elements of the phase III study, and with this framework, Immune plans to submit the final protocol for the phase III study in early 2017 and, upon approval, proceed with conducting a global Phase III Pivotal Overall Survival Study in AML maintenance of response with Ceplene/IL2.
“We are very pleased with the positive outcome of our recent interaction with the FDA. Our path forward to proceed with a pivotal study following regulatory guidance of Ceplene/IL-2 meets our goal to address the urgent unmet medical need for remission maintenance therapy in AML,” stated Monica Luchi, MD, Immune’s CMO.



