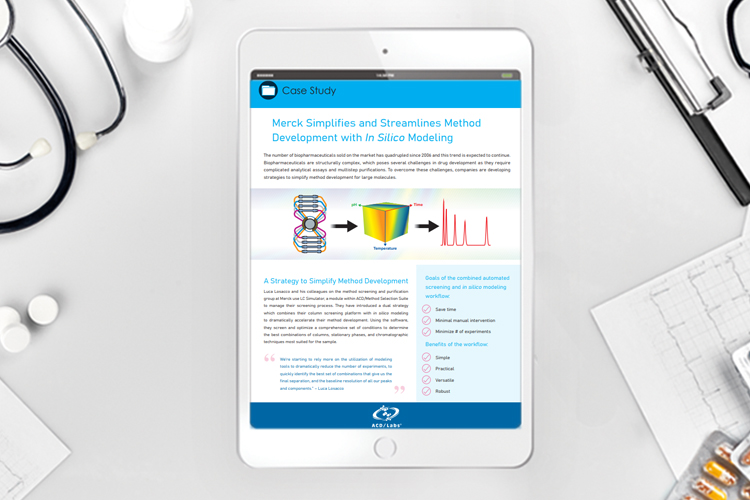
Streamlining bioprocessing for gene therapy
Adopting technological advances in upstream and downstream processes is vital to the gene therapy space, says Kai Lipinski, CSO at ReciBioPharm. Here he explores emerging technology trends and discusses how they can help to overcome key challenges facing gene therapy manufacturers.