Whitepaper: Logistic regression model of the endotoxin standard curve and statistical tests
Posted: 19 December 2018 | Merck | No comments yet
The Monocyte Activation Test (MAT) is used to detect or quantify pyrogenic substances that activate human monocytes.
Pyrogenic substances in pharmaceutical products can induce life-threatening fever reactions after injection into the human body. Therefore, it is a regulatory requirement to test such products for pyrogens to ensure product quality and patient safety.
The MAT has been described as a compendial method for pyrogen detection in the European Pharmacopeia since 2010 (Chapter 2.6.30). It is a test that mimics the human reaction to pyrogens, by using a source of monocytes, which release some interleukins upon activation by pyrogenic substances:
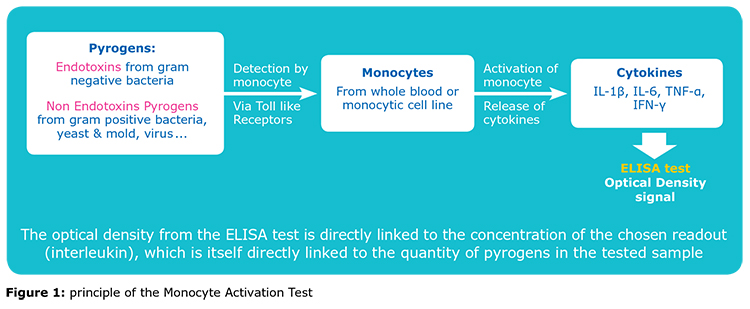

The rest of this content is restricted - login or subscribe free to access
Thank you for visiting our website. To access this content in full you'll need to login. It's completely free to subscribe, and in less than a minute you can continue reading. If you've already subscribed, great - just login.
Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- bi-monthly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Related content from this organisation
- Full data set for new buffer stock blending system freely available
- $65 million expansion to HPAPI manufacturing facility
- QA/QC & Analytical Techniques In-Depth Focus 2020
- QA/QC & Analytical Techniques In-Depth Focus 2020 with a special focus on Chromatography & Microbiology
- Challenges in today’s pharmaceutical formulation
Related topics
Analytical techniques, Assays, QA/QC, Regulation & Legislation










