Whitepaper: Logistic regression model of the endotoxin standard curve and statistical tests
Posted: 19 December 2018 | Merck | No comments yet
The Monocyte Activation Test (MAT) is used to detect or quantify pyrogenic substances that activate human monocytes.
Pyrogenic substances in pharmaceutical products can induce life-threatening fever reactions after injection into the human body. Therefore, it is a regulatory requirement to test such products for pyrogens to ensure product quality and patient safety.
The MAT has been described as a compendial method for pyrogen detection in the European Pharmacopeia since 2010 (Chapter 2.6.30). It is a test that mimics the human reaction to pyrogens, by using a source of monocytes, which release some interleukins upon activation by pyrogenic substances:
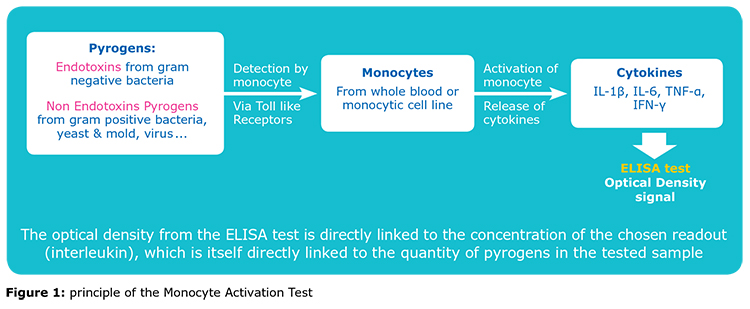

The rest of this content is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- bi-monthly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Related content from this organisation
- Full data set for new buffer stock blending system freely available
- $65 million expansion to HPAPI manufacturing facility
- QA/QC & Analytical Techniques In-Depth Focus 2020
- QA/QC & Analytical Techniques In-Depth Focus 2020 with a special focus on Chromatography & Microbiology
- Challenges in today’s pharmaceutical formulation
Related topics
Analytical techniques, Assays, QA/QC, Regulation & Legislation










