Inhibition of microbial growth in solid dosages at ICH stability storage conditions
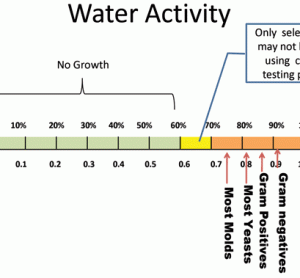
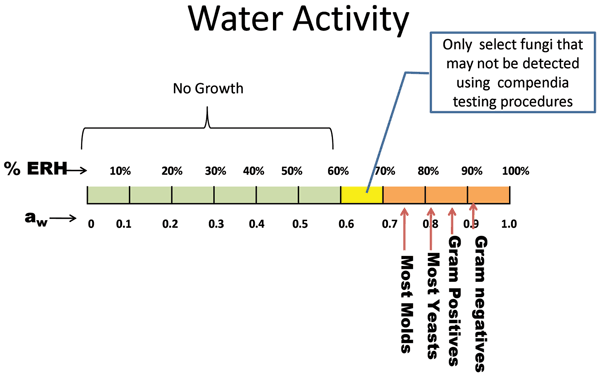
Several intrinsic and extrinsic factors influence microbial growth. Two important…
31 August 2011 | By Linda Skowronsky, Senior Microbiologist, GlaxoSmithKline
Several intrinsic and extrinsic factors influence microbial growth. Two important factors include the presence of available moisture and a supportive temperature. The conditions described in ICH Topic Q1A (R2)1 do not allow the organisms of interest in pharmaceutical solids to grow, due to either an unfavourable temperature or humidity. For…