Inhibition of microbial growth in solid dosages at ICH stability storage conditions
Posted: 31 August 2011 | | No comments yet
Several intrinsic and extrinsic factors influence microbial growth. Two important factors include the presence of available moisture and a supportive temperature. The conditions described in ICH Topic Q1A (R2)1 do not allow the organisms of interest in pharmaceutical solids to grow, due to either an unfavourable temperature or humidity. For this reason, testing either microbial limits or measuring water activity as part of the stability program is considered of little value in determining microbial stability.
An optimised approach for reduced microbial testing to support stability has been proposed2,3. The rationale was given for utilising water activity measurements instead of microbial limit testing on non-aqueous solids with water activity measurements. Many regulatory authorities continue to require microbial limit data although will sometimes accept water activity data to support microbial stability. Quality by Design as described in ICH Q84 emphasises the importance of designing quality into products rather than testing in quality. Therefore, it is important to take a critical view of stability specifications and current testing. Testing typically performed during development is often generated in response to regulators inquiries that consider satisfactory microbial limit and/or water activity data will ensure microbial stability. In the case of materials of low water activity, this conclusion, however, may not accurately reflect in-use conditions.
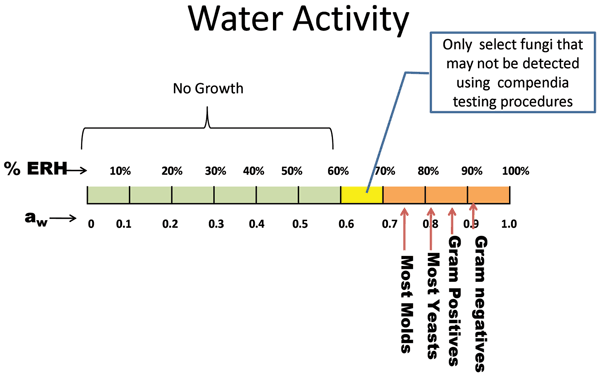

Figure 2 Range of growth at water activity (aw) and relative humidity (%ERH) for various classes of microorganisms
Several intrinsic and extrinsic factors influence microbial growth. Two important factors include the presence of available moisture and a supportive temperature. The conditions described in ICH Topic Q1A (R2)1 do not allow the organisms of interest in pharmaceutical solids to grow, due to either an unfavourable temperature or humidity. For this reason, testing either microbial limits or measuring water activity as part of the stability program is considered of little value in determining microbial stability.
An optimised approach for reduced microbial testing to support stability has been proposed2,3. The rationale was given for utilising water activity measurements instead of microbial limit testing on non-aqueous solids with water activity measurements. Many regulatory authorities continue to require microbial limit data although will sometimes accept water activity data to support microbial stability. Quality by Design as described in ICH Q84 emphasises the importance of designing quality into products rather than testing in quality. Therefore, it is important to take a critical view of stability specifications and current testing. Testing typically performed during development is often generated in response to regulators inquiries that consider satisfactory microbial limit and/or water activity data will ensure microbial stability. In the case of materials of low water activity, this conclusion, however, may not accurately reflect in-use conditions.
Although infrequent, there have been recalls involving contaminated tablets and almost all accounts have been due to mould contamination. It is likely, however, these recalls were due to presence of moulds due to poor GMP’s or raw materials rather than due to actual growth. In order for spoilage to occur, growth must occur and it is this growth that is of interest during stability studies. Organisms in the dynamic phase of growth can degrade products, and some can produce harmful toxins. There are specific requirements necessary for growth which often means finding the right balance between opposing forces of moisture, temperature, pH, etc.
The aim of this article is to provide evidence to support the scientific rationale for elimination of the current practice of microbial testing as well as water activity testing as a measure of microbial stability on solid dosages or other solid drug products. These materials must have an initial water activity (aw) of <0.65, and are stored at or below 65%RH for long term stability, or at temperatures exceeding 30°C during pivotal stability.
Scope
The justification provided in this article applies only to stability testing and is not a justification to eliminate microbial testing at release, as low aw does not necessarily reduce the chance of microbial survival; in fact, in some cases it actually promotes survival.
Supportive rationale
Recent guidance on stability by the World Health Organisation5 does not include microbial testing on stability of tablets, powders and suppositories. Although microbial testing of capsules is listed, the listing is ‘for consideration’. It seems the WHO agrees with the position presented here! In addition, ICH Q1A1 requires testing to be performed on attributes that could change during storage and this testing must be stability indicating.
The stability indicating tests of the Microbial Limits are the enumeration tests to include Total Aerobic Microbial Count (TAMC) and Total Yeast and Mould Counts (TYMC) which are described in Ph. Eur. 2.6.126. The unacceptable ‘change’ would be an increase in numbers of microorganisms that would indicate growth. In order for growth to occur on storage, the following conditions must be met (Figure 1):
1. Organisms must be present and recoverable by compendia methods
2. Sufficient available water in the product must be present
3. Organisms must be able to grow under the storage conditions
Note: The tests for specified organisms are not considered stability indicating tests since these are qualitative tests and presence was found to be absent initially; growth of ANY organism as measured by TAMC/TYMC would be considered a stability failure.
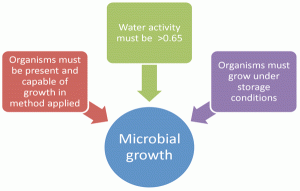

Figure 1 Conditions for detection of microbial growth on storage
Organisms must be present and recoverable by the applied method
In order for growth to occur, organisms must be present and recovery of organisms doesn’t necessarily mean ‘growth’. Conditions needed for survival can be very different from the conditions needed for growth and it is growth, measured by an increase in numbers, that is of interest on storage. The bio-burden, or organisms present in the raw materials that have survived the manufacturing process, are determined in the Microbial Limit tests, as described in Ph Eur 2.6.126 and 2.6.137, at release. The survivors will decrease, remain static, or increase (grow) during storage. As long as growth doesn’t occur, these organisms are not harmful to the product or consumer if within the limits specified and has been determined not to be a health risk. In many cases, however, products made using good quality materials, under good GMP’s, and in some cases a hostile process or ingredients, may contain few or no surviving organisms. Absence of growth on storage could mean the absence of organisms rather than a determination of microbial stability. This is true for any product type.
The Microbial Limit tests will detect most contaminants in raw materials and finished products that could be harmful or cause spoilage. In order to recover these contaminants they must be able to grow aerobically, in the highly aqueous environment provided by the recovery media, and at the temperatures defined in the method. These conditions will not permit growth of anaerobes; also recovery of xerophilic or osmophilic fungi may require specialised media since they prefer a drier environment8,9. The methods also describe the temperature ranges of 30°C-35°C for total aerobic count (bacteria and fungi) and 20°C-25°C for fungi. Although these temperature ranges will detect most organisms, there are certain psychrophilic and thermophilic organisms which will not grow at these temperatures and therefore will not be recoverable.
Obuekwe10 reported 67 per cent of the 22 samples of tablets tested from pharmacies in Nigeria showed bacterial and fungal contamination when viewed by electron microscope; while only 18 per cent showed recovery on agar. There is no information on the viability of the organisms seen visually, and there is no evidence of an actual increase in numbers indicating growth. This does show, however, recovery using the usual methods may not accurately reflect the presence or absence of organisms.
There must be sufficient available water in the product for growth
Most solid dosage forms typically have a low aw of <0.5 if produced at normal temperate conditions of 25°C/45%RH. As described in USP <1112> Application of Water Activity Determination to Non-sterile Pharmaceutical Products11, as long as the aw is <0.60, microbial growth cannot occur since there is insufficient available moisture.
Water activity can be described as the equilibrium relative humidity (ERH)% /100 and measures how tightly bound the water is in a material12,13. However, since moisture migrates from areas of high aw to areas of low aw, a material that has aw =0.5, for example, can pick up moisture from the environment as long as the humidity is, in this case, >50%ERH14. For this reason, although growth is inherently inhibited in solids under normal conditions, growth may be possible if held in an environment of high humidity.
Gram negative organisms as well as some Gram positives such as Bacillus spp., and Streptococci spp., require aw >0.90 to grow. To achieve this amount of moisture, the product would have to be in an environment of >90%ERH. Figure 2 and Table 1 both describe the minimal water activities needed for growth. S.aureus could grow as low as aw =0.83 or 83%ERH in an optimal environment. Most yeasts will grow with a minimum aw = 0.87, and many moulds can grow as low as aw = 0.75. As described earlier, there are some fungi that can grow as low as aw = 0.6, although these may not be recoverable by the methods employed8,9.


Figure 2 Range of growth at water activity (aw) and relative humidity (%ERH) for various classes of microorganisms
Table 1: Temperature and Water Activity needed for Growth
Organism | Temp ˚C | Water Activity | %RH required |
| Bacteria | Optimum | Minimum | Minimum |
| Most spoilage bacteria | 20-30 | 0.90 | 90% |
| Escherichia coli | 37-41 | 0.94 | 94% |
| Pseudomonas aeruginosa | 20-30 | 0.96 | 96% |
| Salmonella spp | 35-37 | 0.93 | 93% |
| Staphylococcus aureus | 35-39 | 0.83 | 83% |
| Yeasts | |||
| Most Yeast | 25 | 0.87 | 87% |
| Candida lipolytica | – | 0.87 | 87% |
| Saccharomyces rouxxi | – | 0.60 | 60% |
| Mold | |||
| Stachybotrys chartarum | 23 | 0.89 | 89% |
| Aspergillus flavus | 40 | 0.78 | 78% |
| Aspergillus fumigatus | 43 | 0.82 | 82% |
| Aspergillus niger | 37 | 0.80 | 80% |
| Aspergillus versicolor | 30 | 0.75 | 75% |
| Penicillium chrysogenum | 25 | 0.75 | 75% |
| Penicillium brevicompactum | 25 | 0.75 | 75% |
S. aureus and Enterobacter sp. survived quite well at low water activity 23%RH (aw= 0.23) and 46%RH (aw =0.46), as the humidity increased up to 0.95% (aw =0.95), survival actually diminished for these bacteria. Above 95%RH, however, rapid growth occurred. This might explain why bacteria are not regarded as problematic spoilage organisms for tablets since they require a lot of moisture and organisms would likely die as the water activity approached the level where growth could occur16.
Most fungi are metabolically active over a broad temperature range; however, high moisture and relative humidity are required for optimal growth17.
When the mould spores of Aspergillus niger were inoculated on lactose/starch tablets at 31°C/75 %RH no growth occurred; however rapid growth was seen at 95%RH18,19. Fungal spores can remain viable for extended periods of time under normal conditions, however for growth and spoilage to occur, high levels of moisture are necessary for sustained periods of time20. This might explain why exposure to periodic rises in bathroom humidity often don’t present mould growth issues. However, other undesirable chemical factors may occur such as loss of physical integrity or drug effectiveness. The environmental increases in water activity can be controlled with desiccants and moisture impermeable packaging as displayed in Figure 3.
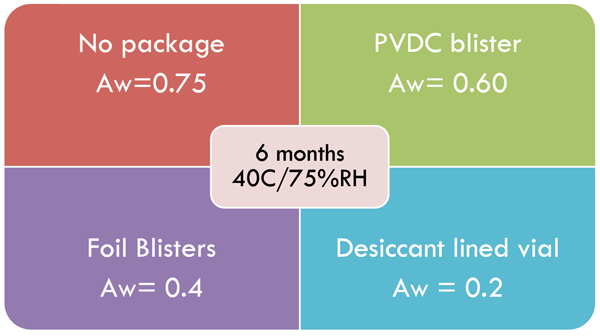
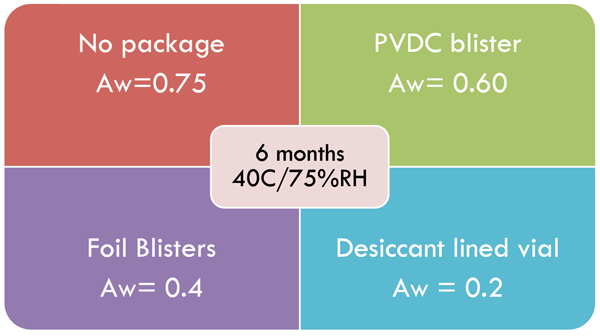
Figure 3 Packaging effects on water activity of a mannitol base lozenge on storage or six months at 40°C/75%RH
Organisms must be able to grow in under the ICH conditions of storage
ICH Q6A Guidelines for Stability describes the storage conditions necessary to ensure stability. For solids to be marketed in Zones 1 & 2 (US & Europe), the long term storage conditions are 25C/60%RH; Zones 3 and 4a require storage at 30C/65%RH; the hot, humid ASEAN countries require long term storage of 30C/75%RH (see Table 2). Other storage conditions listed include accelerated 40C/75%RH.
Like water activity, temperature is another important factor regulating microbial growth; and each organism has a minimum, optimum and maximum temperature for growth. Below the optimum temperature, organisms may not grow as rapidly but will survive, while above the optimum temperature, growth will be reduced and organisms will begin to decline12.
Most organisms relevant to pharma – ceuticals will grow at temperatures between 4°C and 30°C. At temperatures greater than 30°C, some water and environmental contaminants will not grow. Although the pathogenic organisms optimally grow at 37°C, these will suitably grow at 25°-30°C with adequate moisture21. As seen in the stability storage conditions in Table 2, all Zones have a temperature that could support growth of most organisms at their optimum range with the exception of the 40°C accelerated temperature which will not support growth of most environmental isolates. Although some organisms could potentially grow at 40°C, they have to overcome other ‘hurdles’ one being aw in order to initiate growth.
Table 2: Growth Capacity at Stability Storage Conditions for Solids
| 25C / 60% RHZones I & II | No growth – Aw too low |
| 30C / 65% RHZones III & IV 4a | No growth – Aw too low |
| 30 C / 75% RHZone IV b & Accelerated back-up | Growth possible – if certain moulds were present and material was supportive |
| 40C / 75% RHAccelerated | No growth – temperature too high for sub-optimal water activity |
Zones I & II: temperate and subtropical = examples: Canada, UK & US and S. Europe
Zones III & IVa: hot/dry & hot/ humid = examples: Iran, Iraq & Australia, Brazil, Nigeria
Zones 4b: ASEAN countries: Hot/ very humid: examples Philippines, Malaysia, Vietnam, Singapore
The food industry has been utilising the ‘Hurdle’ concept for evaluating microbial risk and growth potential. This technology utilises combined preservative concepts or ‘hurdles’ that microorganisms must overcome in order to grow. These hurdles include temperature, water activity, pH, redox potential, preservatives, etc. A certain amount of effort is required to overcome each hurdle and the more unfavourable the condition, the higher the hurdle and greater effort required22,23. This Hurdle approach might explain why it was described that as the temperature moved away from optimum, more moisture was needed for growth16.
The water activity of a low moisture product on storage at ICH conditions can either decrease with the aid of a desiccant, remain the same which can be accomplished with a moisture impermeable container or package such as foil laminate, or it can increase. The increase can only be as high as the relative humidity of ambient environment. This is demonstrated in Table 3 that shows that tablets stored for two weeks without package at 40°C/75%RH were only able to reach a maximum aw ≤0.75. Conditions for growth at Zones 1 & 2 at 60%RH are therefore intrinsically controlled.
Table 3: Water Activity of some OTC/and Nutritional Health Supplements Stored At 40˚C/75%RH without Packaging.
Samples | Time = 0 | Time = 2 weeks |
| Film Coated aspirin 325 mg | 0.34 * | 0.65 |
| 120 mg naproxin sodium tablet | 0.26 * | 0.70 |
| Calcium Carbonate Chewable Tablet fruit flavor | 0.43 | 0.75 |
| Nicotine Lozenge | 0.04 * | 0.74 |
| Acetominophen Gel Caps PM – 500 mg | 0.45 | 0.74 |
| 500 mg acetominophin/diphenhydramine | 0.48 | 0.69 |
| 25 mg diphenhydramine caplet | 0.24 * | 0.74 |
| Cranberry urinary health tablet- 450 mg | 0.43 | 0.74 |
| 200 mg Ibuprophen caplet | 0.16 * | 0.74 |
| Non-drowsy cold capsule 3.25 mg acetominophen, 15 mg dextromethorphen, 30 mg pseudoephedrin (blister pack) | 0.47 | 0.73 |
| Orlistat- 60 mg capsules | 0.38 * | 0.73 |
* Tablets containing desiccant before storage.
Solid dosages stored at Zones III and IVa conditions at 30°C/65%RH could potentially allow growth of certain organisms such as osmophlic yeasts and xerophilic moulds, however, these organisms require certain ingredients such liquid sorbitol, glycerine and corn syrup to be taken into its cell structure to prevent hydrolysis. Although these may be present in a cough syrup24, they are unlikely to be present in a solid dosage. Furthermore, these organisms may not be recoverable by the usual methods25. Conditions of storage at 30°C/65%RH for these zones therefore also intrinsically control growth. Food is considered safe if aw =0.70 or below1212. The U.S. Food and Drug Administration26 consider foods that have aw < 0.85 to be classified as ‘non-hazardous’. One should then consider storage at or below 70%RH to be safe for storage of solid dosages.
Only the 30°C/75%RH storage for Zone IVb (hot and humid ASEAN countries) could potentially support growth of some moulds as seen in Table 4. These conditions would be minimally favourable for growth of some xerophilic moulds such as Aspergillus and Penicillium species provided other hurdles such as pH, etc were favourable27. This condition may warrant monitoring growth of moulds if survival has been detected initially.
Table 4: Proposed Stability Testing for Microbiology
| ICH Conditions of storageºC / % RH | Microbial LimitsTAMC / TYMC | Water ActivityAw |
| 25 / 60 (Zone I & II) | No | No |
| 30 / 65 (Zone III & Iva) | No | No |
| 30 / 75 (Zones IV b) | Maybe1 | Yes |
| 40 / 75 (Accelerated) | No | No |
1 test only if aw >0.65 and moulds are present at release
Does this mean that the product could not support growth? Under most ICH conditions of storage, the answer would be ‘no’. However, if the product was exposed to changing temperatures when condensation was possible, or the humidity and temperature were optimal, it would be possible for microorganisms to grow. These conditions, however, are not typically part of the stability program for solid oral dosages.
Conclusions
To summarise, microbial limit testing and water activity testing is of little value in predicting microbial stability of solid dosages that have an initial aw <0.65 for the following reasons:
- 60%RH storage conditions will not support growth
- 65%RH storage conditions could support growth but organisms may not be recoverable and these organisms are not relevant to these formula types
- 75%RH could support mould growth; 40°C will not support mould growth at low aw
- 30°C/75%RH could potentially support mould growth if certain moulds were present
- History has shown testing microbial limits and water activity on stability has proved little value to what could actually occur in use
For the reasons described in this article, the elimination of microbial testing and water activity measurements on all solids not to be sold in the very hot and humid environment of Zones IVb is recommended. If 30°C/75%RH storage is scheduled, monitor for growth only if the product has demonstrated moulds at release and if aw >0.65 on storage (see Table 4).It is suggested that resources would be better utilised in acquiring a better understanding of the growth supportive ability of the product in an environment of high humidity >90 per cent or in materials exposed to cycling where condensation is a possibility. Since the only information that could be gathered from testing water activity is package permeability, this should be the rationale behind this testing since the product would not only be susceptible to growth in high humidity, but also to other physical and chemical degradations.
References
1. International Convention on Harmonization (ICH) Q1A (R2) (2003) Stability Testing of New Products and Substances
2. Cundall, A. & Fontana, A. Jr. (2009), Water Activity Applications in the Pharmaceutical Industry Pub. by DHI, p. 223-252
3. Skowronsky, L. (2008) A practical approach to microbial testing to support non-sterile product stability, European Pharmaceutical Review Vol, 13, Issue 6, p.76
4. International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use (ICH) Q8 Product Development (2005)
5. World Health Organization Technical Report Series No. 953, 2009 Annex 2: Stability Testing of Active Pharmaceutical Ingredients and Pharmaceutical Products http://www.who.int/medicines/publications/pharmp rep/pdf_trs953.pdf#page=101
6. European Pharmacopeia 2.6.12: Microbiological Examination of Non-Sterile Products: Microbial Enumeration Tests
7. European Pharmacopeia 2.6.13: Microbiological Examination of Non-Sterile Products: Tests for Specified Organisms
8. Beuchat, L. R. 1982, Influence of Water Activity on Growth, metabolic Activities and Survival of Yeasts and Molds, Journal of Food Protection, Vol 46, No. 2 P. 135-141.
9. Rockland, L. B., Beuchat, L. R. (1987) Water Activity: Theory and Applications to Food, Marcel Dekker, Inc p 158-165
10. Obuekwe, C. O., Obuekwe, I. F., and Rafiq, M (2000) Surface microbial contamination in some commonly available tablet dosage forms Medical Principles and Practices 9: 290-299
11. United States Pharmacopeia/ National Formulary (2011) <1112> Application of Water Activity Determination to Non-sterile Pharmaceutical Products ) The Official Compendia of Standards, U.S. Pharmacopeial Convention, Rockville, Maryland
12. Food and Drug Administration Inspection Guide: Water Activity (aw) in Foods http://www.fda.gov/ICECI/Inspections/InspectionGui des/InspectionTechnicalGuides/ucm072916.htm
13. Banwart, (1989), Basic Food Microbiology Chapman & Hall pp101-113; 136
14. Moir, P, ( 2008),’ Microbial Limit Testing- Reduced/ Replaced by Equilibrium Relative Humidity’ TOPRA Regulatory Rapporteur 5 (4): 14-16
15. Mold and Bacteria Consulting Laboratory What Are Some Of The Mycotoxin Producing Indoor Moulds? May 31, 2005 By MBL http://www.moldbacteriaconsulting.com/2005/05/w hat-are-some-of-mycotoxin-producing.html
16. Bloomfield, S.F., Baird, R., Leak, R.E. and Leech, R (1988) Water Activity and Microbial Survival Chpter 8 sec 8.3 in Microbial Quality Assurance in Pharmaceuticals, Cosmetics, and Toiletries. Ellis Horwood Ltd.
17. Kuhn, D. M. and Ghannoum, M. A. (2003). Indoor Mold, Toxigenic Fungi, and Stachybotrys chartarum: Infectious Disease Perspective. Clinical Microbiology Reviews, 16(1):144–172.Nielsen, K.F,
18. Bos, C.E., van Doorne, H.and Lerk, C.F., Microbiological stability of tablets stored under tropical conditions International Journal of Pharmaceutics Volume 55, Issues 2-3, 15 October 1989, Pages 175-183
19. Holmquest, G. U. Walker, H. W. and Stahr, H. M., (1983), Influence of Temperature, pH, Water Activity and Antifungal Agents on the growth of Aspergillus flavus and A. parasiticus, Journal of Food Science, Vol. 48 p. 778-782
20. Baird, R. and Bloomfield, S. (1996) Microbial Quality Assurance in Cosmetics, Toiletries and Non-sterile Pharmaceuticals 2nd edition, CRC Press, p.36
21. Prince, R. 2001 Microbiology in Pharmaceutical Manufacturing p 82 22. Leistner, L.and Gorris, L.G. M. (1995) Food Preservation by Hurdle Technology, Trends in Food Science and technology Vol.6
23. Rockland, L. B., Beuchat, L. R. (1987) Water Activity: Theory and Applications to Food, Marcel Dekker, Inc p 296-300
24. Gervais, P, Marechal, P.A., Molin, P, (1992) Effects of the Kinetics, of Osmotic pressure Variation on Yeast Viability, Biotechnology and Bioengineering, Vol 40 pp 1435-1439
25. Beuchat, L. (1983) Influence of Water Activity on growth, Metabolic Activities and Survival of Yeasts and Molds. Journal of Food Protection Vol. 46. No 2 p.135-141
26. FDA (2009) Evaluation and Definition of Potentially Hazardous Foods. http://www.fda.gov/ Food/ScienceResearch/ResearchAreas/SafePracticesf orFoodProcesses/ucm094151.htm
27. Gock Melissa A. , Hocking, Ailsa D. , Pitt, John I., Poulos, Peter G. (2002) Influence of temperature, water activity and pH on growth of some xerophilic fungi International Journal of Food Microbiology 2481 (2003) 11 – 19
Linda Skowronsky has 38 years of experience in pharmaceutical/consumer health microbiology which includes over 30 years in her present position at GlaxoSmithKline Consumer Healthcare and seven years with Warner-Lambert Company (now Pfizer and J&J). During this time she has led the microbiological activities from development through the regulatory submission processes of many global OTC drug formulations. She has co-authored chapters in two PDA publications as well as other articles particularly in the areas of water activity and risk assessment. Linda graduated with a BS in Biology from Stonehill College in Massachusetts, and is a certified Medical Technologist from Morristown Memorial Hospital.







