Physiologicaly based pharmacokinetic modelling of transporters in drug discovery and development
3 September 2012 | By Pradeep Sharma and Katherine Fenner, Global DMPK, AstraZeneca R&D
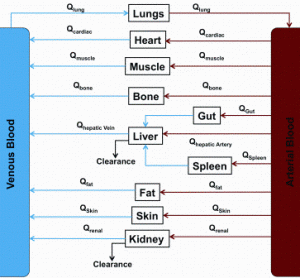
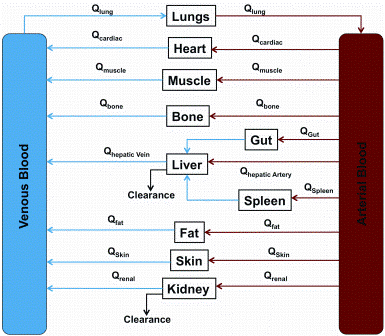
Physiologically based pharmacokinetic (PBPK) models describe the different compartments (tissues) in the body linked via arterial and venous blood flow (Figure 1). The volume of each tissue and blood flows are available from literature data1-5 and PBPK models have been developed for many species including rat, mouse, dog, pig and…