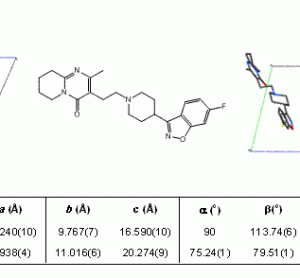
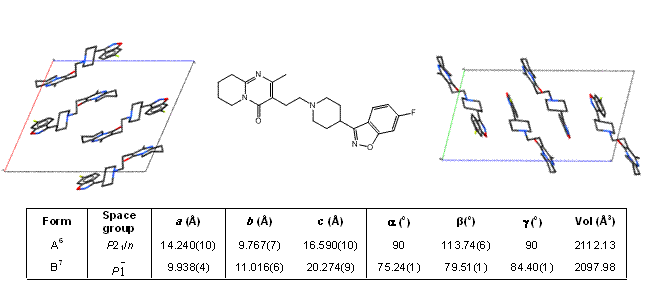
Application Note: Particle characterisation of pharmaceutical powder mixtures (count, size and chemistry)
For the study of a pharmaceutical drug product, particle characteristics (count, size and chemistry) and compound distributions (size and spatial) are among key parameters that strongly determine the quality of the product...