Application of DSC and MDSC in the development of freeze dried pharmaceuticals
Posted: 3 December 2008 | | 4 comments
Freeze drying of pharmaceuticals requires an adequate formulation design to prevent low-temperature, freezing and drying stresses. The goal is to achieve a final product with long storage stability and elegant appearance. To meet these specifications the product temperature must be controlled below the critical formulation temperature during the freeze drying cycle. DSC is an established tool to measure this critical formulation property in the development of freeze dried pharmaceuticals as it allows rapid sample preparation and analysis time. The introduction of modulated DSC (MDSC) by Reading in 1992 has greatly facilitated the interpretation of DSC results. The overlapping transitions in the same temperature range can be distinguished and characterisation of the nature of transitions is facilitated.
Freeze drying: an important drying method for unstable pharmaceuticals
Freeze drying, or lyophilization, is a process in which the solvent, typically water, is transformed into a frozen solid (ice) and subsequently removed by sublimation. Freeze drying is usually performed by applying a vacuum (0.06 – 0.30 mbar) and a very low shelf temperature to enable sublimation. The objective of this procedure is to enhance storage stability of pharmaceuticals, which are unstable in the liquid state1. This applies in particular to biopharmaceuticals like proteins, peptides, vaccines, recombinant DNA, plasmids, etc but also to novel drug delivery systems like nano-particles and liposomes2. In this context it is important to mention that this technique even prevents both chemical (hydrolysis, oxidation, deamidation etc) and physical instability (particularly aggregation) when stabilisers (cryo and lyoprotectants) are added, followed by an adequate freeze drying cycle design3. Cycle-optimisation is of crucial importance to reduce cycle time (typically between 48 and 96 hours), increase product turnover and therefore increase profits. This, however, implies that the product temperature at the sublimation interface must be maintained close to the “critical” formulation temperature during primary drying, but may not exceed this temperature boundary4. Note that literature reports that an increase of product temperature of 1°C can decrease primary drying time up to 13%5. To minimise the risk of losing entire batches it is a common strategy to use rather conservative drying conditions (i.e. cycles are designed far from optimum). Nevertheless, there is an increased demand of this drying technology as lyophilization is often the only procedure to make new drugs available to the patients. Additionally, freeze drying offers crucial benefits compared to other methods, (e.g. spray-drying) with regard to:
- The formation of a highly porous cake-structure
- Filling of exact dosages in final container
- The residual moisture in the final product can be controlled
- It is easy to meet the requirement of a sterile product6.
Since 2000, the FDA (Food and Drug Administration) encourages optimisation of freeze drying cycles in the laboratory and in pharmaceutical manufacturing, focusing on an improvement in GMP (Good Manufacturing Practice) and quality control7. The following examples illustrate why DSC and MDSC became such indispensable tools in the development of freeze dried pharmaceuticals, techniques which determine physico-chemical properties of the formulation.
Formulation and stability
Formulation development of biopharmaceuticals as well as for colloidal drug delivery innovations is challenging since lyophilization generates a variety of stresses8. In general, excipients are added to a active component to overcome these instability issues. Excipients can be categorised in bulking agents, stabilisers, buffer, surfactants and “miscellaneous”, depending on their role in the formulation3,8. Here, the physico-chemical state of each individual substance is of paramount importance for the choice of the drying parameters. For instance, bulking agents should provide cake stability and an attractive appearance. Mannitol and glycine belong to this class, as they tend to readily crystallise9,10. However, it is essential to ensure complete crystallisation of those excipients during the freezing step to avoid stability problems of the final product. In contrast, stabilisers must be kept in the same physico-chemical state like the active pharmaceutical ingredient (API) to achieve a physical “mixture” in which stabiliser and API may interact8. Frequently used disaccharides like sucrose and trehalose are well characterised and were investigated to act as both cryo and lyoprotectants. In particular, buffer systems can be critical as they have the capability to significantly affect the critical formulation temperature in case they remain amorphous. Thus, crystallisation is desired for this group of excipients. In turn, in a few cases crystallisation could also cause instability. For example, phosphate buffer show a severe pH shift that is based on selective crystallisation of a buffer component and finally leads to inactivation of the API during the freezing step1. In many cases, the API formulation remains completely amorphous or partially crystalline. As a result, a detailed analytical investigation of the amorphous fraction, its glass transitions and recrystallisation tendency is required.
Importance of the critical formulation temperature
The critical formulation temperature signifies the highest allowable product temperature during primary drying and is represented by the eutectic temperature (Te) for crystalline and glass transition temperature of the maximally freeze concentrated solute (Tg’) or the collapse temperature (Tc) for amorphous structures4. In primary drying the temperature of the product is determined by the chamber pressure (Pc) and the shelf temperature (Ts)11. Note that the product temperature at the ice sublimation interface (i.e. the phase boundary between frozen product and dried structure) is of particular importance. Ice sublimes and permeates vertically through the dried upper product layer. As a consequence, pores are formed which result from the solid fraction of the formulation. The freezing behavior of an aqueous amorphous solution has been described in the literature as follows12:
- After supercooling of the solution and subsequent ice nucleation, the temperature of the solution increases up to the equilibrium freezing point.
Further decrease in temperature leads to freeze-concentration of the solute until a high viscous liquid (rubber-like) is obtained. During this phase, the matrix will not crystallise but is stabilised based on the high viscosity. At the temperature Tg’ ice crystal growth is completed and the solution is fully transformed into a solid (glassy state). It should be noted that in this glassy state an immense fraction of water is dispersed (e.g. sucrose up to 20%12). If the product temperature increases above Tg’, viscosity of the amorphous solute will decrease, resulting in a change in product morphology. This change in product morphology may be classified in “microcollapse” (barely visible and most notably inside of the cake), “shrinkage” (volume contraction, but preservation of the porous structure) and (full) “collapse” (overall loss of porous structure)13. The presence of cake shrinkage or collapse may be problematic as this may lead to rejection of the final product, even if the product is fully active, see Figure 1.


Not only prolonged reconstitution times and incomplete dissolution, but also increased water content and low glass transition temperatures may compromise storage temperature (e.g. room temperature vs. refrigerated) and shelf life of the drug.
Application of DSC in formulation and process development
Application of DSC in freeze drying can be divided into two steps. First, thermal analysis serves as a screening method of diverse liquid formulations. The objective is to receive as much information as possible about the individual physico-chemical behavior of the excipients in the mixture during the freezing procedure and therefore determine the critical formulation temperature as exact and representative as possible. These results determine significantly the subsequent strategy for the freeze drying process design11. The following list summarises the application of DSC for liquid formulations:
- Determination of Tg’ using different heating rates. A fast heating rate increases sensitivity (e.g. to detect weak transitions), a low heating rate improves resolution (e.g. multiple transitions within a narrow temperature range must be separated)
- Evaluation of the heating rate on Tg’ information14
- Evaluation of heating rate on crystallisation behavior for individual excipients.
- Thermal treatment of the sample (often denoted as “annealing”15), conducted above Tg’ to investigate the onset of crystallisation and study the maximum grade of crystallisation
- Influence of excipient concentration on Tg’ in a multicomponent mixture as well as their crystallisation behavior in a given mixing ratio during heating (e.g. mannitol, glycine or buffer in a mixture with stabilisers)
- Investigation of a potential phase separation between excipient and API or between excipient and excipient during the freezing step16,17.
The measurement of physico-chemical properties after freeze drying (solid system) is essential to predict storage stability. Here, DSC might be useful for:
- Determination of Tg of the lyophilized product, to evaluate storage conditions (e.g. ambient temperature or refrigerated). As a rule of thumb, freeze-dried products should be stored at least 50°C below the first Tg in the formulation18
- Investigation of recrystallisation of excipients during storage
- Analyzing the grade of crystallisation in combination with X-ray powder diffractometry
- Evaluation of residual moisture by Karl-Fischer titration19 and with this the influence on Tg (note that water act as a plasticiser)20.
The above mentioned points influence stability and therefore quality of the pharmaceutical product. An increased molecular mobility of an API (e.g. a protein or peptide) in the amorphous matrix, based on inadequate storage conditions and/or low glass transition temperatures may cause degradation and subsequently inactivation of the product.

Application example for liquids: Determination of Tg’
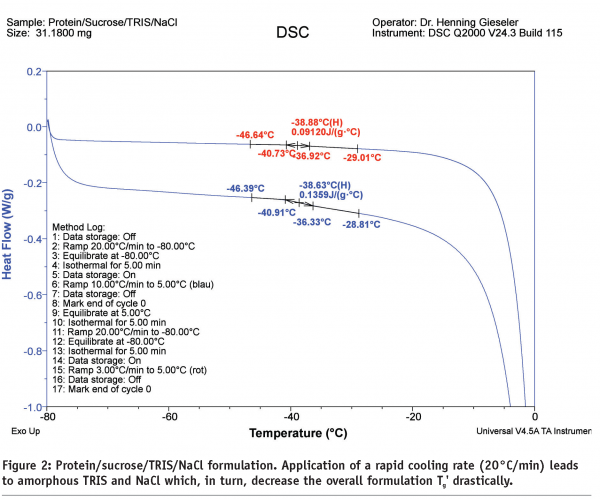
A prerequisite for rational freeze drying process design is an accurate determination of Tg’ if the formulation remains completely amorphous. Typically, a standard DSC with fast cooling and heating rates is performed first to reduce effective development time. Typically, several formulation compositions for a single API are tested. In addition, statistical experimental designs (Design of Experiments, DoE) are used which require multiple DSC measurements. However, a fast cooling (or heating) rate may lead to a less representative interpretation of results. For example, a buffer or a salt would remain amorphous if a high cooling rate of 10-20°C/min is used. This would greatly affect the overall formulation Tg’.
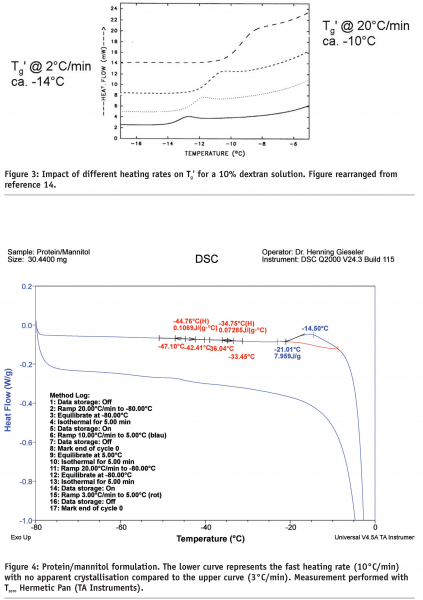
Figure 2 illustrates a protein/sucrose/TRIS/NaCl mixture where the TRIS buffer (Tg’ of the pure amorphous component: -65°C) and NaCl (isotonicity modifier, Tg’ of the pure amorphous component: < -60°C) remain amorphous during the freezing procedure. Note that this formulation would be expected to have a formulation Tg’ of about -30 to -25°C. A second problem with regard to representativeness of the Tg’ data, is the heating rate, see Figure 3.

In this context it should be noted that freeze drying is a very slow process with initial cooling rates of 0.5-1°C and a very slow product temperature increase during ice sublimation (a few degrees over multiple hours)1,6,11. Applying an additional slow scan rate during DSC measurement is indispensable to gain useful information for the freeze-drying process.
Figure 4 depicts the above mentioned issues (scan rate and crystallisation) with regard to a protein/mannitol formulation (weight ratio 1:3). At first fast freezing of 20°C/min results in fully amorphous mannitol. Afterwards two heating rates were chosen (10°C/min. and 3°C/min.). During the fast heating rate the mannitol fraction has not enough time to crystallise. On the contrary a heating rate of 3°C/min leads to an exothermic event at (onset) -21°C indicating re-crystallisation of the bulking agent. Furthermore, these results help to estimate annealing-conditions for the described formulation. As a general rule annealing should be performed not only to facilitate crystallisation of certain compounds but also to receive homogeneous ice crystallisation resulting in more homogeneous drying conditions21.
Application example for liquids: Gordon-Taylor equation
The Gordon-Taylor equation is well known in polymer sciences to calculate glass transition for binary, ternary etc mixtures, assuming that all amorphous fractions are ideally mixable. In freeze dry formulation development this equation offers a great benefit, an estimate for Tg’ of a new formulation20. The goal is to achieve a favorable Tg’ for freeze drying process (> -35°C). Tg’ of the mixture (Tg-MIX’, in Kelvin) can be described by the following equation:
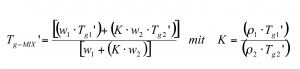
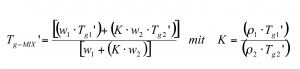
Where Tgx’ (Kelvin) is the glass transition of the individual amorphous compound, wx the weight fraction of amorphous compounds in the total mixture, K is a constant and ρx the real density of the compounds. For simple formulations, this calculation is in accordance with Tg’/Tg obtained from a DSC measurement. However, DSC measurements of protein formulations containing a lyoprotectant have been reported previously to clearly deviate from the Gordon-Taylor calculation13 which underlines that this calculation can not replace a DSC measurement. Also, the Gordon-Taylor equation can not be recommended when developing semi-crystalline formulations (e.g. mannitol based formulations). For example, in a formulation which also (besides mannitol) contains an amorphous stabiliser, mannitol may or may not crystallise, at least not quantitatively. As a result, the weight fraction of the amorphous mannitol is unknown and the calculated Tg’ of the Gordon-Taylor equation would be ambiguous. Again, the real density of a component and the exact amorphous fraction of each component must be known to obtain helpful information from this equation20.
Summary: Application of DSC for liquid formulations
The results obtained from DSC-measurements provide essential information for the subsequent freeze drying process design. First, a formulation may be classified as predominantly amorphous, semi-crystalline or crystalline. The following points should then be considered:
Mainly amorphous
- Estimate Tg’ via Gordon-Taylor equation during initial formulation development: get information about individual compounds with regard to glass transitions
- Tg’ < -40°C is not useful for freeze drying. Consider Tg’ increasing additives (e.g. dextran, cyclodextrin, polymers, etc.) to be added to the mixture
- In particular for high concentrated protein-solutions: initially a fast heating rate should be applied (20-30°C/min) to identify weak transitions, but the shift in temperature must be considered. Low ramp rates (2-3°C/min) should be tested, parallel to the used of the MDSC mode (if available)
- Maximum freezing temperature can be critical, as Tg’ for some excipients (e.g. buffer < -60°C) is extremely low. It is recommendable to start with preliminary scans at temperatures around -80°C.
Semi-crystalline
- Estimate Tg’ via Gordon-Taylor equation during initial formulation development, but crystalline fractions must completely crystallise. Otherwise the resulting Tg’ will be much lower than expected (or calculated)
- Apply slow freezing-rates to facilitate crystallisation of the crystalline fraction; use thermal treatment (“annealing”) steps in your DSC experiment to study crystallisation potential
- Evaluate appropriate “annealing” temperature of the formulation and required annealing time
- Drying above Tg’ is possible, but stability studies (+40°C / 75rH / ≥ 4 weeks) on the final product must proof that there is no negative impact on the active component.
Mainly crystalline
- Tg’ of amorphous phase is negligible, but stability testing should be performed (+40°C / 75rH / ≥ 4 weeks)
- Note that inadequate freezing could generate an “artificial” Tg’, but herein the critical formulation temperature is Te.
Application example for solids: Estimation of residual moisture
As mentioned above, the residual moisture content in lyophilized products is an important quality characteristic due to the plasticising effect of water on the amorphous matrix. Even small amounts of water may result in a clear decrease of Tg with the consequence that storage conditions are affected. Tg of the solid should be high since labile pharmaceuticals like antibiotics or vitamins often show inactivation at elevated molecular mobility22,23. The “gold standard” with regard to residual moisture in a freeze dried cake is less than 1%. However, some proteins were reported to be more stable with higher residual moisture contents (e.g. tPA, hGH).
Sometimes it is impossible to determine residual moisture by Karl-Fischer titration, because ingredients of a sample interact with the measuring medium, e.g. ascorbic acid in the sample would react with iodine. Thermal analysis, like TGA and DSC, can overcome this limitation.
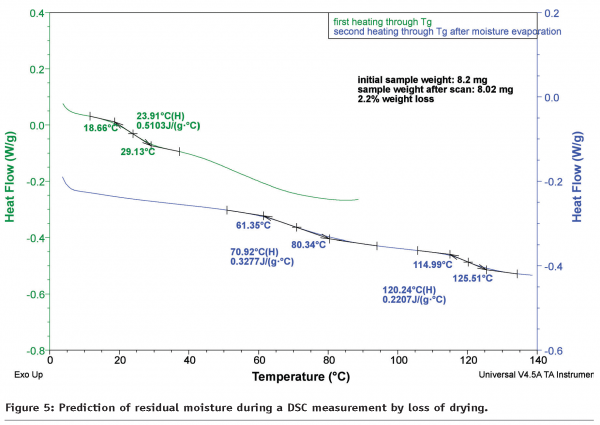

In Figure 5 the amount of residual moisture is estimated by loss of drying. Before DSC measurements were applied, the sample in Figure 5 was equilibrated at 30% relative humidity for 12 hours. Afterwards 8 mg of the sample was weighed into a Tzero Hermetic Pan (TA Instruments) and then sealed. Determination of Tg with unknown residual moisture content was performed using a scan rate of 3°C/min. Then, a pin-hole was punched in the lid and the sample pan re-used to evaporate the residual moisture at 105°C over a 30 minute period. Subsequently, Tg was determined again by using the same ramp rate applied in the first scan. Tg was increased from ca. 24°C to 71°C accompanied by a mass loss of about 2.2%. A prerequisite for this method is to investigate residual moisture contents between 0% and 3% as these values are relevant for freeze-dried products. Note that it is recommended to perform TGA measurements in advance to the DSC experiment to evaluate optimum equilibration temperature and time.
Summary: Application of DSC for freeze dried products (solids)
As described above, determination of Tg for freeze dried pharmaceuticals is a crucial measurement to evaluate storage conditions or correlate stability of a given product. Additional analytical techniques should be used to gain more insight into crystallisation behavior (X-ray powder diffraction) and residual moisture content (Karl-Fischer titration). For mainly crystalline products, identification of Tg becomes difficult (weak transition) but it is less relevant for storage temperature conditions. For amorphous or semi-crystalline products, residual moisture content is important. Here, Karl-Fischer and Tg-values from literature facilitate interpretation of DSC-results with regard to Tg of the lyophilized product. A heating rate of 10°C/min provides a good overview about transitions and examination of a temperature range from 0°C to 100°C is sufficient. If weak transitions occur, thermal aging (isothermal 10-20°C below the expected Tg) could improve sensitivity and or resolution. Furthermore, choosing of a smaller sample mass or lower scan rate may improve resolution, but it should be noted that an increased resolution is generally at the expense of sensitivity and vice versa (increase of sample mass or scan rate will result in improved sensitivity to detect weak transitions).
MDSC technique facilitate interpretation of thermal transitions
Sometimes either above mentioned limitations of DSC or baseline stability makes it difficult to interpret thermal transitions. Since Reading (1992) introduced Modulated DSC (MDSC) these limitations are overcome by the use of a sinusoidally oscillating heating rate. The key benefit of MDSC is the ability to separate overlapping thermal transitions which occur in the same temperature range (e.g. an enthalpic relaxation peak sometimes superimposes a Tg and even an experienced operator struggles to interpret the data correctly)24-26. In comparison to DSC, MDSC provides simultaneously both increased sensitivity (MDSC eliminates baseline curvature) and resolution, due to implementation of two heating rates (modulated temperature program) during one measurement. Note that MDSC can not replace DSC, as this technique exhibits disadvantages (time consuming, since the underlying heating rate is much lower than for DSC and complexity of choosing modulation parameter). Generally before using the MDSC mode an operator should consider not to choose modulation parameter which exceeds heating and cooling capability. Moreover, long periods should be chosen to prevent thermal gradients within the sample. As a rough rule, at least six modulations through any transition are recommended. In addition, low sample mass results in minimum thermal gradients which allows faster periods and larger amplitudes27. On the other hand, high sample mass minimises baseline curvature, resulting in increased sensitivity. To give an idea of first MDSC-measurement amplitude of 0.159°C, period of 30 seconds and a scan rate of 2°C/min is a good compromise (as used for a TA Instruments Q1000 and Q2000) but the choice of modulation temperature is always specific to the instrument.
Conclusion
DSC has evolved among other analytical tools in the development of freeze-dried pharmaceuticals into an indispensable method for determination of the critical formulation temperature, and is therefore essential for a rational freeze drying process design. Introduction of MDSC has greatly improved the capability to interpret thermal transitions since the technology improves both sensitivity and resolution. However application of a modulated temperature program requires some experience, because an inaccurate choice of modulation parameter will inevitably lead to false interpretation of a measurement.
References
- M.J. Pikal. 2002. Freeze Drying. Encyclopedia of Pharmaceutical Technology, Marcel Dekker, New York.
- J.J. Schwegman, L.M. Hardwick, M.J. Akers. 2005. Practical Formulation and Process Development of Freeze-Dried Products. Pharm Dev Technol, 10: 151-173.
- W. Wang. 2000. Lyophilization and development of solid protein pharmaceuticals. International Journal of Pharmaceutics, 203: 1-60.
- M.J. Pikal, S. Shah. 1990. The collapse temperature in freeze drying: Dependence on measurement methodology and rate of water removal from the glassy phase. International Journal of Pharmaceutics, 62: 165-186.
- M.J. Pikal. 1995. Use of laboratory data in freeze drying process design: Heat and mass transfer coefficients and the computer simulation of freeze drying. J Parent Sci Technol 39:115–138.
- H.R. Costantino and M.J. Pikal. 2005. Lyophilization of Biopharmaceuticals. AAPS Press series, Biotechnology: Pharmaceutical Aspects,Vol. 2.
- FDA/ORA Compliance Policy Guide. Sub Chapter 490.100. Process Validation Requirements for Drug Products and Active Pharmaceutical Ingredients Subject to Pre-Market Approval, 2006: www.fda.gov/ora/compliance_ref/cpg/ default.htm.
- J.F. Carpenter, M.J. Pikal, B.S. Chang, T.W. Randolph. 1997. Rational Design of Stable Lyophilized Protein Formulations: Some Practical Advice. Pharm Res 14(8): 969-975.
- R.K. Cavatur et al. 2002. Crystallization Behavior of Mannitol in Frozen Aqueous Solutions. Pharm Res 19(6): 894-900
- A. Pyne, R Surana, R. Suryanarayanan. 2002. Crystallization of Mannitol below Tg’ during Freeze-Drying in Binary and Tenary Aqueous Systems. Pharm Res 19(6): 900-908.
- X. Tang, M.J. Pikal. 2004. Design of Freeze-Drying Processes for Pharmaceuticals: Practical Advice. Pharm Res 21(2):191-200
- S. Nail, L. Gatlin.1993. “Freeze Drying: Principles and practice“. In: A. Avis, A. Liebermann, L. Lachmann, editors. Pharmaceutical dosage forms, Vol. 2. Marcel Dekker, New York: 163 -333.
- E. Meister, H. Gieseler. 2008. Freeze-Dry Microscopy of Protein/Sugar Mixtures: Drying Behavior, Interpretation of Collapse Temperatures and a Comparison to Corresponding Glass Transition Data. J. Pharm. Sci. (published online: Sep 29 2008).
- L-M. Her, S.L. Nail. Measurement of Glass Transition Temperatures of Freeze-Concentrated Solutes by Differential Scanning Calorimetry. Pharm Res, Vol. 11, No. 1, pp. 54-59, 1994.
- X. Lu, M.J. Pikal. 2004. Freeze-Drying of Mannitol-Trehalose-Sodium Chloride-Based Formulations: The Impact of Annealing on Dry Layer Resistance to Mass Transfer and Cake Structure. Pharm Dev Technol.: 9 (1): 85-95.
- A.M. Padilla, M.J. Pikal, E.Y. Shalaev. 2007. Phase Separation in Freeze-Dried Amorphous Solids: Evaluation of Suitable Techniques for Detection. Proc. AAPS National Biotec Conference, San Diego, CA, USA.
- T.W. Randolph. 1997. Phase Separation of Excipients during Lyophilisation: Effects on Protein Stability. J Pharm Sci 86(11): 1198-1203.
- B.C. Hancock, S.L. Shamblin. 2001. Molecular mobility of amorphous pharmaceuticals determined using differential scanning Calorimetry. Thermochimica Acta, Volume 380, Issue 2.
- FDA Guideline for the Determination of Residual Moisture in Dried Biological Products, 2008. http://www.fda.gov/CBER/gdlns/ moisture.htm
- B.C. Hancock, G. Zografi. The Relationship Between the Glass Transition Temperature and the Water Content of Amorphous Pharmaceutical Solids. Pharm Res, Vol. 11, No. 4, pp. 471-477, 1994.
- J.A. Searls, J.F. Carpenter, T.W. Randolph.2001. Annealing to Optimize the Primary Drying Rate, Reduce Freezing-Induced Drying Rate Heterogeneity, and Determine Tg’ in Pharmaceutical Lyophilization. J Pharm Sci, 90 (7): 872-887.
- M.J. Pikal, D. Rigsbee, M.L. Roy, D. Galreath, K.J. Kovach, B. Wang, J.F. Carpenter, M.T. Cicerone. 2008. Solid State Chemistry of Proteins: II. The Correlation of Storage Stability of Freeze-Dried Human Growth Hormone (hGH) with Structure and Dynamics in the Glassy Solid. J Pharm Sci 97(12): 5106-5121.
- M.J. Pikal, D. Rigsbee, M.L. Roy. 2008. Solid State Stability of Proteins III: Calorimetric (DSC) and Spectroscopic (FTIR) Characterization of Thermal Denaturation in Freeze Dried Human Growth Hormone (hGH). J Pharm Sci, 97(12): 5122-5131.
- P.G. Royall, D.Q.M. Craig, C. Doherty. 1998. Characterisation of the Glass Transition of an Amorphous Drug Using Modulated DSC. Pharm Res 15(7): 1117-1121.
- D.Q.M. Craig, P.G. Royall. 1998. The Use of Modulated Temperature DSC for the Study of Pharmaceutical Systems: Potential Uses and Limitations. Pharm Res 15(8): 1152-1153.
- E. Verdonck, K. Schaap, L.C. Thomas. 1999. A discussion of the principles and applications of Modulated Temperature DSC (MTDSC). International Journal of Pharmaceutics 192: 3-20.
- V.L. Hill, D.Q.M. Craig, L.C. Feely. 1999. The effects on experimental parameters and calibration on MTDSC data. International Journal of Pharmaceutics 192: 21-32.





I would like to have the full paper
Hi Ouassyla, Thanks for your message. This online article is the full version of the paper.
I would like to have the full paper
Hi Simone, viewing this paper (and all of the papers published on the website) is easy, simply register here and you’ll be able to access the full paper.