Four years and counting for automation
Posted: 20 July 2006 | | No comments yet
As a classically trained electrophysiologist, I shall always remember my first encounter with automated patch clamp at Essen Instruments in June 2002. Essen Instruments had just developed the IonWorks HT, an instrument that can record currents from 48 cells simultaneously and perform up to 384 recordings with a drug addition step in a single 45-minute experiment1,2. This accomplishment coincided with the start of a revolution in the field of ion channel drug discovery.
As a classically trained electrophysiologist, I shall always remember my first encounter with automated patch clamp at Essen Instruments in June 2002. Essen Instruments had just developed the IonWorks HT, an instrument that can record currents from 48 cells simultaneously and perform up to 384 recordings with a drug addition step in a single 45-minute experiment1,2. This accomplishment coincided with the start of a revolution in the field of ion channel drug discovery.
As a classically trained electrophysiologist, I shall always remember my first encounter with automated patch clamp at Essen Instruments in June 2002. Essen Instruments had just developed the IonWorks HT, an instrument that can record currents from 48 cells simultaneously and perform up to 384 recordings with a drug addition step in a single 45-minute experiment1,2. This accomplishment coincided with the start of a revolution in the field of ion channel drug discovery.
During the past four years several automated patch clamp instruments have been commercialised, covering a large range of applications and pertinent to various ion channel families. This article reviews the current applications of automated patch clamp in ion channel drug discovery and its coverage of the ion channel genome. Recent articles have reviewed the various automated electrophysiology instruments in detail3,4 as well as the past, present and future of automated patch clamp5.
Bringing patch clamp electrophysiology to critical phases of ion channel drug discovery
In contrast to most drug targets, ion channels have benefited from having a technology, patch clamp electrophysiology, that allows direct, real-time measurements of the activity of single to thousands of ion channels at rest or responding to various stimuli or drugs. This technology therefore is the gold standard method for examining ion channel activity and is preferred over indirect methods. But patch clamp electrophysiology has also hampered the discovery and development of novel ion channel drugs due to its very low throughput and high costs. Until the advent of automated patch clamp technologies, significant bottlenecks limited ion channel drug discovery, such as the optimisation and validation of biological reagents and the validation of hits from high throughput or focused screens.
Non-electrophysiological assays such as ion flux assays or fluorescence-based assays may be used to assess the functional expression of ion channels in various cells. However several of these assays give non-linear measurements of ion channel activity. In addition, electrophysiological recordings are necessary for the characterisation and validation of novel reagents, from verifying the voltage-dependence and kinetics of heterologously expressed voltage-gated channels to examining state and use-dependent modulation of channel activity by pharmacological standards. With transient, as well as stable, expression systems, only a limited number of expression conditions or cell clones are typically examined by conventional electrophysiology owing to its low throughput and serial nature. Automated patch clamp, with its relative ease-of-use and higher throughput, has helped develop and validate better ion channel reagents. First, automation has simplified or eliminated many steps required to perform patch clamp recordings, such as manually selecting cells and obtaining seals. Therefore patch clamp electrophysiology is now more accessible to non electrophysiologists. Nanion’s bench top Port-a-Patch instrument, for example, can be found in several molecular biology groups where it is used to examine the functional expression of new ion channel reagents. This instrument is the world’s smallest patch clamp setup with a recording station foot print covering about a quarter of the area of this page6,4. In addition, its design is very simple, with a recording station that holds a planar patch clamp chip, to which cells and solutions can be added directly by the user. Cell positioning, sealing, perfusion and recording are all controlled by the Port-a-Patch software. This instrument allows single cell recordings with the possibility to perfuse extra and intracellular recording solutions. Second, a higher throughput has helped identify better ion channel reagents by increasing the number of conditions or clones that can be tested in a single day by up to ten fold. IonWorks HT, the parallel automated patch clamp instrument mentioned earlier, has revolutionised reagent validation and characterisation for ion channels, allowing the testing of up to 100 clones or expression conditions in a single day. On that instrument, as many as 12 different expression conditions can be tested in a single experiment with up to 32 data points per condition7,8. Thus this platform provides, in addition to a higher throughput, a number of data points per experiment sufficient for statistical analysis.
The area where automated electrophysiology has probably had the largest impact is the validation of hits following high to medium throughput screens performed with indirect assays. Traditionally, few hit exemplars (<100 total) from different chemical series are selected for validation in conventional patch clamp assays. Presently, with automated electrophysiology systems that can provide up to ~3,000 data point per day, thousands of hits can be validated by electrophysiology in a very short time. Thus most drug discovery companies have now integrated automated patch clamp into their hit validation strategies. Various instruments are used for this purpose, from instruments with a medium throughput (hundreds of data points per day) that allow gigaseal recordings and solution exchange such as the PatchXpress 7000A, the Qpatch 16, or the Flyscreen 8500 to the IonWorks Quattro with up to 3,000 data points per day, but with lower seal resistances and no perfusion capability4.
Another major application of automated patch clamp electrophysiology is the profiling of compounds in ion channel assays, particularly for liability testing versus the cardiac potassium channel hERG. Prior to entering clinical trials, all drug candidates must be tested for their potential to prolong the cardiac QT interval. Since most drug-induced QT prolongations have been attributed to hERG channel inhibition, there is a huge demand for the early profiling of molecules in functional hERG assays. Pharmaceutical companies as well as contract research companies have now turned to automated patch clamp technologies to eliminate the bottleneck associated with conventional hERG electrophysiology. Several platforms have been validated for hERG screening and are now used routinely for hERG profiling, including the PatchXpress 7000A, the Qpatch 16, and the IonWorks HT4,5,8,9,10,11.
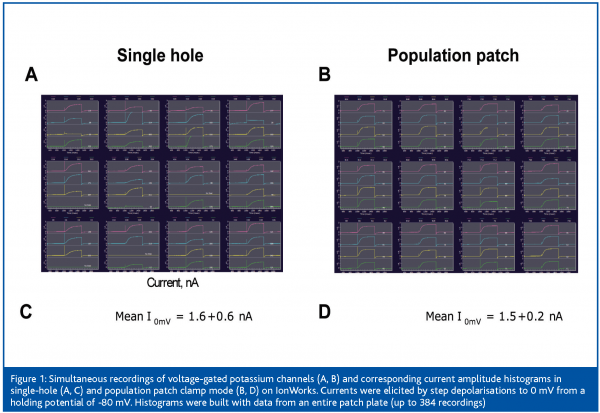
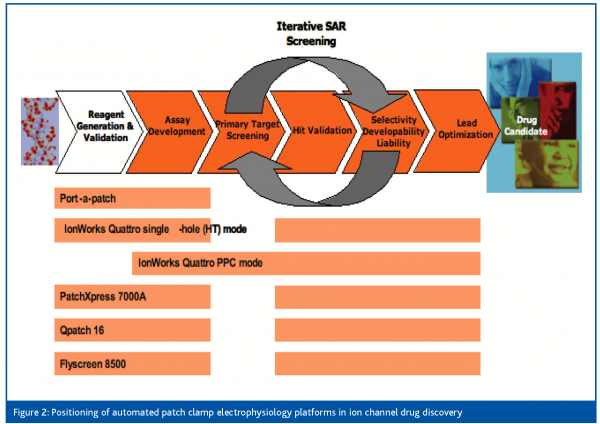
Finally, with the introduction of the population patch clamp technology by Molecular Devices Corporation last year, electrophysiology has become a primary assay platform for the screening of ion channel focused sets containing up to 20,000 compounds. Population patch clamp (PPC) consists of measuring ensemble currents from a population of cells12. This technology is derived from the IonWorks planar patch clamp technology where a single cell seals onto a single aperture per patch plate well. For PPC recordings, each patch plate well has not 1, but 64 apertures to which cells can seal onto. A single voltage clamp amplifier sums up currents from as many as 64 cells per well. This results in greater data consistency and a probability of getting 384 recordings per 384-well plate very close to 1. Figure 1 illustrates the reduced variability of the recordings and higher success rates of PPC electrophysiology. Consequently, the PPC platform is suitable for screening in 384-well format. However, because each well’s parallel resistance is the inverse of the sum of 64 conductances, pre-requisites for successful PPC recordings are high seal rates and good individual seal resistances12. Last year Amgen scientists reported a screen of approximately 12,000 compounds performed on the IonWorks Quattro PPC platform in less than 10 working days and with a failure rate around 0.5%13. Thus, from building new ion channel reagents to compound profiling, automated electrophysiology is a very valuable technology for ion channel drug discovery. Figure 2 summarises the positioning of the various commercialised instruments in the different phases of drug discovery. Another dimension to consider though is the application of automated electrophysiology to various channel types which, as described below, can be limited by technical factors.
Automated patch clamp electrophysiology and the ion channel genome
Ion channels are defined by their ability to transport ions at a very fast rate, often more than 106 ions per second, without the expenditure of cellular energy14. However ion channels belong to very diverse families and are regulated by a wide range of stimuli. Both chemical and physical energy can dictate their opening and closing and channels have been divided into ligand-gated, voltage-gated and constitutively active or ‘leak’ channels. Conventional patch clamp electrophysiology can accommodate all these channel types due to the very high degree of flexibility built into its hardware and software. However automated systems have various limitations that restrict their application to certain channel types.
A major limiting factor is the time required to completely exchange solutions during automated recordings. The fastest automated systems report exchange rates of 50 to 100 ms or 50 to 100 times slower than fast perfusion systems used in manual patch clamp experiments4,15. Slower solution exchanges rule out recording from very fast ligand-gated ion channels such as the P2X1 and nicotinic alpha 7 acetylcholine receptors. The latter, for example, open and close in less than 100 ms in the continued presence of the agonist acetylcholine15. IonWorks HT and Quattro are the only platforms where compounds are added instead of perfused and recordings are not performed during drug addition. The shortest time between drug addition and recordings on the Quattro platform is 8 seconds7. Thus, the application of this platform to ligand gated channels should be severely limited. Nevertheless, successful recordings from slow ligand-gated channels, such as Trpv6, have been reported on that platform16. Therefore, the IonWorks platform could in principle be used for reagent validation of slow ligand-gated channels, if not for compound profiling.
Another challenge for automation of electrophysiology recordings is the handling of leak currents and performing adequate leak subtraction for voltage-independent or leak channels recordings. A trained electrophysiologist can easily distinguish true leak currents attributed to ion channel activity from non-specific increases in the leakiness of the recordings due to problems such as seal failure or cell death. However, an instrument cannot make this distinction, unless experimental conditions have been carefully crafted to discriminate the different types of leak. Several groups have started to address this issue and have reported significant progress toward developing leak channel assays on the IonWorks and Qpatch automated platforms. For example, the IonWorks Quattro, with population patch clamp recordings, has enabled the development of assays for IK and SK channels17. In those assays, extracellular K is kept low so that a true K conductance linked to channel opening will have a negative reversal potential and non-specific leak will have a reversal potential near 0 mV. By recording currents at 0 mV, only channel activity is measured. In addition, summing up and averaging currents from multiple cells with PPC has made recordings from those channels highly reproducible and fit for pharmacological testing.
So, with the exception of very fast ligand-gated ion channels which represent a significant fraction of the ion channel genome, automated electrophysiology is applicable to a wide range of ion channels, from voltage-gated potassium channels to GABA-gated channels5. However automated patch clamp may not be as critical for some ligand-gated ion channels. An important factor to consider is the availability of high quality non electrophysiological assays for ligand-gated ion channels. For example, highly predictive fluorescence-based calcium assays have been developed for many calcium-permeable ligand-gated channels. For those channels, while having the option to automate electrophysiology is attractive, it is not as critical as for voltage-gated ion channels.
In the four years since the introduction of automated electrophysiology in industrial laboratories, automated patch clamp instruments have found a place in all phases of ion channel drug discovery, from reagent generation and characterisation to drug candidates safety profiling. Current instruments have some limitations and relatively high costs, but the quality and high information content of electrophysiological recordings are worth the investments made in this technology. In addition, this field is maturing and innovative, second generation instruments are already starting to appear. One sure sign is the introduction of population patch clamp by Molecular Devices Corporation last year, allowing primary ion channel screens to be performed by electrophysiology.


References
- Schroeder K.; Neagle B.; Trezise D.J.; and Worley J. Ionworks HT: a new high-throughput electrophysiology measurement platform. J. Biomol. Screen. 8(1):50-64, 2003.
- Kiss, L.; Bennett P.B.; Uebele V.N.; Koblan K.S.; Kane S.A.; Neagle B. and Schroeder K. High throughput ion-channel pharmacology: planar-array-based voltage clamp. Assay Drug Dev. Technol. 1(1 Pt 2):127-35, 2003.
- Moore, P. Ion channels and stem cells. Nature 438:699-702; 2005.
- Comley, J. Automated patch clamping, setting a new standard for early hERG. Drug Discovery World, 7(1):62-79, 2006.
- Mathes, C. Qpatch: the past, present and future of automated patch clamp. Expert Opin. Ther. Targets 10(2):319-327, 2006.
- www.nanion.de
- www.moleculardevices.com
- Guthrie, H.; Livingston, F.S.; Gubler, U. and Garripa, R. A place for high-throughput electrophysiology in cardiac safety: screening hERG cell lines and novel compounds with the IonWorks HT system. J. Biomol. Screening. 10(8):832-840; 2005.
- Dubin, A.E.; Nasser, N.; Rohrbacher, J.; Hermans, A.N.; Marrannes, R.; Grantham, C.; Van Rossem, K.; Cik, M.; Chaplan, S.R.; Gallacher, D.; Xu, J.; Guia, A.; Byrne, N.G. and Mathes, C. Identifying modulators of hERG channel activity using the PatchXpress planar patch clamp. J. Biomol. Screen. 10(2):168-81, 2005.
- Guo, L. and Guthrie, H. Automated electrophysiology in the preclinical evaluation of drugs for potential QT prolongation. J. Pharmacol. Toxicol. Methods. 52(1):123-35, 2005.
- Bridgland-Taylor, M.H; Hargreaves A.C.; Easter A.; Orme A.; Henthorn D.C.; Ding M.; Davis A.M.; Small B.G.; Heapy C.G.; Abi-Gerges N.; Persson F.; Jacobson I.; Sullivan M.; Albertson N.; Hammond T.G.; Sullivan E.; Valentin J.P. and Pollard, C.E. Optimisation and validation of a medium-throughput electrophysiology-based hERG assay using IonWorksHT. J. Pharmacol. Toxicol. Methods, 2006, in press.
- Finkel, A.; Wittel, A.; Yang, N.; Handran, S.; Hughes, J. and Costantin, J. Population patch clamp improves data consistency and success rates in the measurement of ionic currents. J. Biomol. Screening. 2006, in press.
- See 2005 automated electrophysiology webinar by Joseph McGivern at http://www.moleculardevices.com
- Hille, B. Ion channels of excitable membranes. 3rd ed. Sinauer, 2001
- Delbono, O.; Gopalakrishnan, M.; Renganathan, M.; Monteggia, L. M.; Messi, M. L. and Sullivan, J.P. Activation of the recombinant human alpha7 nicotinic acetylcholine receptor significantly raises intracellular free calcium. J. Pharm. Exp. Ther. 280(1):428-438, 1997.
- See 2006 automated electrophysiology webinar by Martin Goslin at http://www.moleculardevices.com
- See 2006 automated electrophysiology webinar by Derek Trezise at http://www.moleculardevices.com




