Drug-drug interactions: tools for drug transporter protein studies
Posted: 30 July 2009 | Dr Maria Vlaming, Project Leader, TNO Quality of Life; Dr Jeroen DeGroot, Team Leader of Kinetics and Metabolism, TNO Quality of Life; Dr Miriam Verwei, Project Leader within the field of Kinetics and Metabolism, TNO Quality of Life and Dr Heleen Wortelboer, Senior Scientist and Project Leader within the PharmacoKinetics and Metabolism Team, TNO Quality of Life | No comments yet
Drug transporters are membrane proteins involved in the uptake or efflux of drugs by several tissues such as the intestine, liver, kidney and brain. They can have a significant impact on the pharmacokinetics of endogenous and exogenous compounds. Also, co-administered drugs or nutrients can influence the transporter activity which may lead to changes in the pharmacokinetics of drugs and, as a result, possibly to reduced efficacy or increased toxicity (so called drug-drug or drug-nutrient interactions). For this reason the regulatory authorities US Food and Drug Administration (FDA) and European Medicines Agency (EMEA) request data on the effects of novel drugs on transporter protein activity.
Drug transporters are membrane proteins involved in the uptake or efflux of drugs by several tissues such as the intestine, liver, kidney and brain. They can have a significant impact on the pharmacokinetics of endogenous and exogenous compounds. Also, co-administered drugs or nutrients can influence the transporter activity which may lead to changes in the pharmacokinetics of drugs and, as a result, possibly to reduced efficacy or increased toxicity (so called drug-drug or drug-nutrient interactions). For this reason the regulatory authorities US Food and Drug Administration (FDA) and European Medicines Agency (EMEA) request data on the effects of novel drugs on transporter protein activity.
Drug transporters are membrane proteins involved in the uptake or efflux of drugs by several tissues such as the intestine, liver, kidney and brain. They can have a significant impact on the pharmacokinetics of endogenous and exogenous compounds. Also, co-administered drugs or nutrients can influence the transporter activity which may lead to changes in the pharmacokinetics of drugs and, as a result, possibly to reduced efficacy or increased toxicity (so called drug-drug or drug-nutrient interactions). For this reason the regulatory authorities US Food and Drug Administration (FDA) and European Medicines Agency (EMEA) request data on the effects of novel drugs on transporter protein activity.
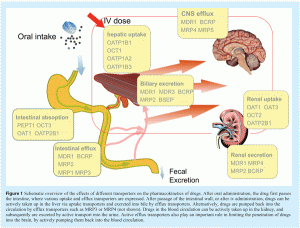
Transporter proteins can be divided into two families, namely the uptake transporters (Solute-Linked Carrier, SLC family) and the efflux transporters (ATP-binding cassette, ABC family), and are expressed in cells of several organs, such as the liver, small intestine and kidney, where they influence the absorption and elimination of their substrates from the body (see Figure 1 on page 48)1-3. Transporter proteins have wide substrate specificities and can therefore influence the pharmacokinetics of many drugs. For example, the most widely studied ABC transporter, P-glycoprotein (P-gp, MDR1) in the intestinal epithelium can affect the intestinal absorption of the immunosuppressant drug cyclosporin A after oral administration by actively pumping the drug back into the intestinal lumen4. Actually, 30% of the variability in plasma Cmax of cyclosporine A in humans can be attributed to inter-individual variation in intestinal P-gp levels5. Besides organs involved in absorption and elimination, drug transporters are also expressed in important tissues such as brain, testis and placenta, often limiting the uptake of drugs into these organs6.
The impact of transporters on drug pharmacokinetics has led to increasing interest in these proteins from the pharmaceutical industry. As observed with drug-metabolising enzymes, inter-individual differences in the expression of specific transporters (e.g. due to polymorphisms or mutations) and interactions of transporters with food components or co-administered drugs can cause changes in the exposure of patients to drugs, possibly leading to adverse effects such as increased toxicity or reduced efficacy of a drug7. It is therefore, of great importance to investigate whether newly developed drugs are transported by, or interact with, transporters.
Transporter-related drug-drug interactions in the clinic
The evaluation of a possible drug-drug interaction potential of candidate drugs has become an integral part of drug development. Since the mid-1990s, several guidance documents of the FDA and EMEA have been released, reflecting the importance to determine possible interactions of newly developed drugs with other (co-administered) drugs due to drug-metabolising enzyme interactions8. Over the past few years it has become clear that besides metabolism, active transport of a drug by transporter proteins can be an important factor in possible drug-drug interactions7-9. Because transporter proteins have saturable binding sites, co-administered drugs may inhibit transporters and can thereby influence the uptake or efflux of another drug. This can lead to reduced excretion or metabolism of drugs and as a result increased internal exposure, which may lead to drug induced toxicity. Furthermore, if a drug is given as a pro-drug, inhibition of uptake transporters (for example in the liver) could prevent metabolism to its active form, leading to reduced efficacy of the drug. On the other hand, stimulation of transporters by a drug is also possible10-12. This could cause increased elimination of drugs when they are co-administered with drugs that stimulate transporters. In this case, the drug will be less effective than when it would have been given alone. In the clinic, a range of transporter-related drug-drug interactions, which in some cases led to severe toxicities or reduced efficacy of drugs, have been reported7.
Transporter proteins are often not only involved in the translocation of drugs, but also pump endogenous compounds over the cell membrane2,13. It is therefore important to note that drugs affecting transporter activity may also influence the physiological function of these transporters. For example, the efflux transporter multidrug resistance-associated protein 2 (MRP2) is involved in the biliary excretion of bilirubin and glutathione, and is also involved in determining bile-salt independent bile flow2. Drug-related inhibition of MRP2 may therefore lead to severe cholestasis and hyperbilirubinemia. This is, for example, suggested for the immunosuppressant drug cyclosporin A and the antibiotic midecamycin, which both have cholestatic potential14,15. Besides MRP2, many other transporters are also involved in physiological processes, again illustrating the importance of studying interactions of drugs with these transport proteins.

In 2006, the FDA published the first draft guidance in which (besides metabolism) the relation between active transport and possible drug-drug interactions was indicated. The knowledge on transporter proteins and their impact on drug-drug interactions was, however, still limited at that time, and drug interaction studies was mainly focused on one transporter protein, P-gp. Nowadays, more than 350 different transport proteins have been described of which about 40 were shown (at least in vitro) to be involved in drug transport. In 2008, a special workshop on transporters was organised by the FDA, and it was recommended that for all newly marketed drugs in oncology and inflammatory disease possible transporter-related drug-drug interactions should be investigated. A new FDA guidance regarding this issue is expected within the coming months. Many in vitro and in vivo methods are currently available to study transporter related drug-drug interactions. The most widely used models to date are discussed below.
In vitro models to study transporter related drug-drug interactions
Due to the increasing interest in transporter proteins an increasing number of methods and models to study drug-transporter interactions were also developed. The method that should be used depends on the specific question one would like to answer, as well as on the physicochemical nature of the drug that is under investigation. In vitro transporter assays are usually carried out with either intact cells or isolated cell membranes over expressing the transporter of interest. The advantage of in vitro assays is, besides obvious ethical issues, that they can be performed with relatively high throughput and low costs as compared to in vivo assays.
In vitro assays to study interactions with efflux transporters
Bi-directional transport assays
In bi-directional transport assays polarised epithelial cells are cultured on a filter insert that separates an apical and basolateral compartment (see Figure 2). With this set-up the influence of a transporter on the actual flux of a drug from the apical to the basolateral side of the cell monolayer and vice versa can be studied in detail. This is the most widely used in vitro assay to investigate whether a drug is a substrate for a specific efflux transporter like the ABC transporters P-gp, and breast cancer resistance protein (BCRP). Alternatively, one can also study the influence of the drug of interest on the transport of a model substrate to study potential drug-drug interactions.
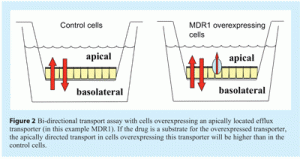

Cell lines that are mainly used for this type of assays consist of cells that polarise in vitro such as transfected MDCKII or LLC-PK cells overexpressing the transporter of interest. The human carcinoma cell line Caco-2 is also often used to study the transport of drugs in combination with inhibitors of the transporter proteins of interest. The advantage of this set-up is that Caco-2 cells are considered a good model for the translocation of drugs over the human intestinal epithelium, expressing considerable levels of a range of transporters. The disadvantage however is, that Caco-2 cells express multiple transporters and that the inhibitors available for use in these studies show limited specificity16. It is therefore often difficult to draw solid conclusions on which specific transporter is actually involved in the observed transport. Recently, additional test systems have been explored using the specific knockdown of the ABC transporter BCRP in Caco-2 cells using shRNA17. It is clear that for these systems evaluation of the expression levels of both the knocked down transporter as well as other transporters is very important.
In general, as these assays use intact and polarised epithelial cells expressing the human transporter, they are probably the in vitro models that will come closest to the in vivo situation. A disadvantage, however, is that for a drug to be transported out of the cell, it first has to be taken up into the cells. Especially with very hydrophilic compounds this is often a problem, as passive diffusion over the cell membrane hardly occurs. Therefore, to study actual binding and transport of this type of compounds, it is usually better to perform a study with inside-out vesicles expressing the efflux protein of interest (see below). An alternative to circumvent this problem is the use of double-transfected cell lines that express both an uptake and an efflux transporter18. However, the uptake transporter necessary for the specific substrate is often not known and it therefore will likely take some effort to determine which specific cell line would be needed.
Vesicular uptake assay
If a compound is hydrophilic and the necessary uptake transporters are not expressed in the cell line used, inside-out vesicles are often used to investigate the interaction of a drug with specific efflux transporters. Inside-out vesicles are derived from the plasma membrane of cell lines (usually the insect cell line Sf-9 or human HEK 293 cells) that over express the efflux transporter of interest. With this assay the active uptake of a drug into the inside-out vesicles expressing this transporter is studied. Alternatively, the interaction of a drug with the uptake of a model substrate of the transporter can be studied to investigate potential drug-drug interactions.
ATP-ase assays
In ATP-ase assays one makes use of the fact that efflux (or ATP-binding cassette, ABC) transporters need ATP for their activity. In this assay, membranes containing the transporter are incubated with a drug. ATP hydrolysis is then used as a measure for the activity of the transporter. When addition of the drug changes the amount of ATP hydrolysis, this indicates an interaction of the drug with the transporter. This assay is very simple and therefore easy to use in a high throughput set-up. However, a disadvantage is that only interactions with the transporter are detected, whereas actual transport of the substrate is not measured. Therefore, based on effects on ATPase activity it is impossible to draw solid conclusions with respect to the nature of the drug-transporter interaction (whether this is transport, inhibition, stimulation etc.).
In vitro assays to study interactions with uptake transporters
Cellular uptake assays
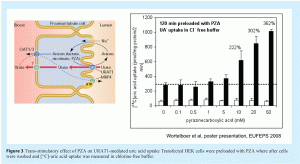
The most commonly used assays to study interactions of a drug with uptake transporters are cell based methods employing cells that over express the uptake transporter of interest. These cell models are ideally based on cell lines from human origin (e.g. HEK 293 cells) as plasma membrane lipid composition can affect transporter function19. However, also cells from other origins (for example CHO, MDCKII, murine cells or Xenopus oocytes) are often used. The cells (and their mock-transfected controls) are plated on 24- or 96-well plates and incubated with the drug under investigation. The time and concentration dependent uptake of the drug or the effects of the drug on the uptake of a model substrate are subsequently analysed. This is a very convenient way to study drug-drug interactions with uptake transporters. One should, however, take into account that uptake transporters can be simporters, antiporters or exchangers, indicating co-transport, anti-transport or exchange of ions for their activity. Presence or absence of these ions in the medium, or absence of ion exchange proteins in the cells may therefore be limiting factors for the detection of transport by these proteins. As an example, the human uric acid transporter (URAT1) is expressed at the apical membrane of proximal tubule cells and functions as an exchanger, which takes up urate from the primary urine in exchange for intracellular anions. As such, this transporter could be trans-stimulated by enhanced levels of intracellular anions, such as pyrazinecarboxylic acid, the metabolite of the antituberculose drug pyrazinamide (see Figure 3). An appropriate study design for this type of studies is therefore of utmost importance.

In vivo models to study transporter related drug-drug interactions
Although many in vitro assays are available nowadays to study drug-transporter interactions, it is obvious that the effect of a transporter on the pharmacokinetics of a drug will not only depend on the direct interaction potential between transporter and drug. In the body many factors (e.g. metabolism, tissue distribution of the transporter, presence of endogenous compounds etc.) can influence drug-transporter interactions. To gain insight in the in vivo effect of the drug-transporter interactions, various models are available. In combination with the wide range of analytical techniques such as imaging, microdialysis and gall bladder/urinary bladder cannulations, these models provide useful tools to study the interactions between drugs and transporters in vivo.
Drug-drug interaction studies in laboratory animals
If in vitro studies suggest interactions between a new drug and one or more transporters, the actual in vivo effect of this drug on the pharmacokinetics of known transporter substrates can be studied by co-administration of these compounds to an appropriate animal model. Following this approach, one can investigate whether the drug affects the pharmacokinetics of other drugs, the so-called drug-drug interactions. If the new drug is expected to be a substrate of a transporter, the effect of specific inhibitors of this transporter on the pharmacokinetics of the drug can be determined as well. A disadvantage of these types of inhibition studies in animals is that the inhibitors are usually not very specific and may also influence other processes in the body. It is therefore often difficult to draw solid conclusions on the exact mechanism involved when an effect of the inhibitor is detected. Furthermore, species-specific differences in function and activity of transporters exist, so the results may not always be relevant for the human situation. On the other hand, an advantage of this type of studies is that they can be performed in practically every animal model and therefore are easy to incorporate in the ADME studies routinely performed during drug development. This will lead not only to an additional dataset from standard ADME studies to be used in drug development but also to a better interpretation of the pharmacokinetic profiles found in these animal studies.
Transporter knockout and humanised mice
Now that transporters are drawing more and more attention, also increasing numbers of mouse models for in vivo transporter studies have been generated. Examples are Mdr1a/1b (P-gp) knock-out mice which have already been shown to be of great value in studies on the in vivo effect of the ABC transporter P-gp2-6. However, there are some drawbacks in the use of these mouse models. First of all, the deletion of one transporter can lead to functional take-over by, or upregulation of, other transporters which could transport the substrate as well. In this case the effect of the investigated transporter may be underestimated as no effect of the deletion of the transporter on the pharmacokinetics of the drug will be found. Therefore, recently also many transporter combination knockout mice (mice in which several transporters are deleted) have been generated20. These are good models to study the pharmacokinetics of drugs that are substrates of more than one transporter. Still, although the tissue distribution and substrate specificity of ABC transporters is in general quite comparable to man, there are clearly species-differences. To tackle this problem nowadays “humanised” transporter mice (mice in which the murine transporter is replaced by the human homologue) are also being generated21. In combination with transporter knockout mice these are very useful models to study the interaction of drugs with specific human transporters in vivo.
Conclusions and perspectives
Transporter issues have become of more and more interest, especially as a range of previously unexplained drug-induced toxicities were shown to be caused by drug-transporter interactions7-9. It has therefore become clear that it is important to carefully study possible drug-transporter interactions in the drug development process. However, as many transporters can influence the pharmacokinetics of a drug, it is often difficult to decide which transporter should be studied. On the other hand, studying every transporter known would be very time consuming and costly. Therefore, to study drug-transporter interactions it would be advisable to determine the tests that should be performed based on already existing data on the drug of interest such as in vivo pharmacokinetics in animals, or even data from first-in-human studies. Of course, which transporters are of interest to study also depends on the site of action of the drug and their degree of expression. For example for CNS drugs it would be advisable to study interactions with BCRP and P-gp, as these transporters are expressed at the blood-brain barrier.
Even though many models have been generated to study transporters in vitro and in vivo (animal studies), and although they appear very useful to predict transporter-related questions, it still is difficult to predict the effects in the human body. Transporter expression and activity in animal or cell-based models may differ from the situation in the human body. The next challenge in the transporter field will therefore be the translation of results from transporter models to the human situation. This extrapolation step could be made using a Physiologically Based PharmacoKinetic (PBPK) model22. Attempts are currently being made to realise this, although many hurdles still have to be taken. Next to the transporter activities, the expression of transporters in the different organs of humans should also be described by the PBPK model. More research is needed to describe all the transporter-related processes in detail in a PBPK model and to determine how to incorporate the data from the in vitro or in vivo transporter assays. This will likely be very helpful for the prediction of internal drug levels and related effects in humans, and to determine specifically who should be treated with a drug at which dosage and who should definitely not.
References
- Oostendorp,R.L., Beijnen,J.H. and Schellens,J.H. The biological and clinical role of drug transporters at the intestinal barrier, Cancer Treat.Rev., 35: 137-147, 2009.
- Borst,P. and Elferink,R.O. Mammalian ABC transporters in health and disease, Annu.Rev.Biochem., 71: 537-592, 2002.
- Shitara,Y., Horie,T. and Sugiyama,Y. Transporters as a determinant of drug clearance and tissue distribution, Eur.J.Pharm.Sci., 27: 425-446, 2006.
- Fricker,G., Drewe,J., Huwyler,J., Gutmann,H. and Beglinger,C. Relevance of p-glycoprotein for the enteral absorption of cyclosporin A: in vitro-in vivo correlation, Br.J.Pharmacol., 118: 1841-1847, 1996.
- Lown,K.S., Mayo,R.R., Leichtman,A.B., Hsiao,H.L., Turgeon,D.K., Schmiedlin-Ren,P., Brown,M.B., Guo,W., Rossi,S.J., Benet,L.Z. and Watkins,P.B. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine, Clin.Pharmacol.Ther., 62: 248-260, 1997.
- Schinkel,A.H. and Jonker,J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview, Adv.Drug Deliv.Rev., 55: 3-29, 2003.
- Aszalos,A. Drug-drug interactions affected by the transporter protein, P-glycoprotein (ABCB1, MDR1) II. Clinical aspects, Drug Discov.Today, 12: 838-843, 2007.
- Huang,S.M., Strong,J.M., Zhang,L., Reynolds,K.S., Nallani,S., Temple,R., Abraham,S., Habet,S.A., Baweja,R.K., Burckart,G.J., Chung,S., Colangelo,P., Frucht,D., Green,M.D., Hepp,P., Karnaukhova,E., Ko,H.S., Lee,J.I., Marroum,P.J., Norden,J.M., Qiu,W., Rahman,A., Sobel,S., Stifano,T., Thummel,K., Wei,X.X., Yasuda,S., Zheng,J.H., Zhao,H. and Lesko,L.J. New era in drug interaction evaluation: US Food and Drug Administration update on CYP enzymes, transporters, and the guidance process, J.Clin.Pharmacol., 48: 662-670, 2008.
- Zhang,L., Zhang,Y.D., Strong,J.M., Reynolds,K.S. and Huang,S.M. A regulatory viewpoint on transporter-based drug interactions, Xenobiotica, 38: 709-724, 2008.
- Zimmermann,C., van de,W.K., van de,S.E., Wagenaar,E., Vens,C. and Schinkel,A.H. Species-dependent transport and modulation properties of human and mouse multidrug resistance protein 2 (MRP2/Mrp2, ABCC2/Abcc2), Drug Metab Dispos., 36: 631-640, 2008.
- Pilorget,A., Demeule,M., Barakat,S., Marvaldi,J., Luis,J. and Beliveau,R. Modulation of P-glycoprotein function by sphingosine kinase-1 in brain endothelial cells, J.Neurochem., 100: 1203-1210, 2007.
- Ozvegy,C., Varadi,A. and Sarkadi,B. Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutation, J.Biol.Chem., 277: 47980-47990, 2002.
- van Herwaarden,A.E. and Schinkel,A.H. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins, Trends Pharmacol.Sci., 27: 10-16, 2006.
- Akashi,M., Tanaka,A. and Takikawa,H. Effect of cyclosporin A on the biliary excretion of cholephilic compounds in rats, Hepatol.Res., 34: 193-198, 2006.
- Horikawa,M., Kato,Y., Tyson,C.A. and Sugiyama, Y. Potential Cholestatic Activity of Various Therapeutic Agents Assessed by Bile Canalicular Membrane Vesicles Isolated from Rats and Humans Drug Metab Pharmacokin., 18 : 16-22, 2003
- Cnubben,N.H., Wortelboer,H.M., van Zanden,J.J., Rietjens,I.M. and van Bladeren,P.J. Metabolism of ATP-binding cassette drug transporter inhibitors: complicating factor for multidrug resistance, Expert.Opin.Drug Metab Toxicol., 1: 219-232, 2005.
- Zhang,W., Li,J., Allen,S.M., Weiskircher,E.A., Huang,Y., George,R.A., Fong,R.G., Owen,A. and Hidalgo,I.J. Silencing the breast cancer resistance protein expression and function in caco-2 cells using lentiviral vector-based short hairpin RNA, Drug Metab Dispos., 37: 737-744, 2009.
- Ishiguro,N., Maeda,K., Saito,A., Kishimoto,W., Matsushima,S., Ebner,T., Roth,W., Igarashi,T. and Sugiyama,Y. Establishment of a set of double transfectants coexpressing organic anion transporting polypeptide 1B3 and hepatic efflux transporters for the characterization of the hepatobiliary transport of telmisartan acylglucuronide, Drug Metab Dispos., 36: 796-805, 2008.
- Hegedus,C., Szakacs,G., Homolya,L., Orban,T.I., Telbisz,A., Jani,M. and Sarkadi,B. Ins and outs of the ABCG2 multidrug transporter: an update on in vitro functional assays, Adv.Drug Deliv.Rev., 61: 47-56, 2009.
- Kruh,G.D., Belinsky,M.G., Gallo,J.M. and Lee,K. Physiological and pharmacological functions of Mrp2, Mrp3 and Mrp4 as determined from recent studies on gene-disrupted mice, Cancer Metastasis Rev., 26: 5-14, 2007.
- Stanley,L.A., Horsburgh,B.C., Ross,J., Scheer,N. and Wolf,C.R. Drug transporters: Gatekeepers controlling access of xenobiotics to the cellular interior, Drug Metab Rev., 41: 27-65, 2009.
- Fenneteau,F., Turgeon,J., Couture,L., Michaud,V., Li,J. and Nekka, F. Assessing drug distribution in tissues expressing P-glycoprotein through physiologically based pharmacokinetic modeling: model structure and parameters determination. Theor Biol Med Model. 6:2, 2009.




