Medicine delivery by drone – implications for safety and quality
Posted: 20 November 2019 | Dr Paul G Royall (King's College), Patrick Courtney (tec-connection) | No comments yet
Drones that transport medicines to emergency situations, urban environments or geographically-challenging locations are increasingly being utilised to improve delivery, however, there are few studies on their impact on drug stability. Here, Paul Royall and Patrick Courtney explore the implications of drone delivery on the safety and quality of medicines.


Credit: IKON IMAGES / JOSH MCKIBLE / SCIENCE PHOTO LIBRARY
To date, the quality of medicines ensured by adherence to a set of guidelines described variously in the British pharmacopoeia and other regulatory documents worldwide.1 Coherence is managed internationally by structures such as the International Committee on Harmonisation (ICH) and has expanded to cover packaging, shipping, security of the supply chain, etc.2 At the same time, there has been considerable interest in the use of robotic systems in pharmacy supply chain logistics, from raw materials and manufacture to pharmacies and patients.3 This technology is advancing fast in terms of performance, range and cost effectiveness compared to traditional logistical means (plane, truck, motorcycle, etc).
There is also a huge demand for cost effective and secure transportation of many other medicinal materials; for example, blood for transfusion, organs for transplant and clinical samples for testing and pathology.4,5 As many hospital services have been centralised or facilities have expanded into new regions, the logistical requirements and transportation of these materials is an ever-growing challenge.


Figure 1: Examples of the available UAVs for medicines transportation.
The quality and stability of a biological sample is sensitive to the length of time it is in transit;6 thus, geographically-challenging locations requiring long and circuitous routes and urban areas that are vulnerable to unpredictable journey times due to excessive congestion in rush-hour traffic, makes the transportation of blood units, organs and other clinical samples difficult within their required arrival times. Uncrewed aerial vehicles (UAV) or drones are a potential option to circumvent this barrier (Figure 1).
Aerial medicine delivery
The use of drones is developing rapidly and there is an urgent need to establish the framework and tools to ensure appropriate handling and confidence in their use for the delivery of medical products. Conventional stability testing methodologies need to be expanded to include the unique stresses that may be encountered during drone transportation, such as vibration, g-force, rapid changes in pressure, humidity and temperature excursions, which may impact the critical material attributes of the medicines. Furthermore, the impact of recent geopolitical transitions within Europe, ie, Brexit, will result in changes to long-standing medical supply chains.8 Thus, in order to maximise availability and avoid stockpiling and the resultant danger of medicines being stored inefficiently and potentially past their sell-by dates in the UK, UAVs offer a good solution, post Brexit.
The state-of-the-art technology enables a UAV to be flown beyond the visual line of sight (BVLOS) while maintaining communication between a ground pilot and the vehicle. However, current civil aviation regulations are lagging9 and in only a few cases, where exceptions have been made, have pilot studies been able to demonstrate the true potential of these over-the-horizon delivery missions (Table 1).
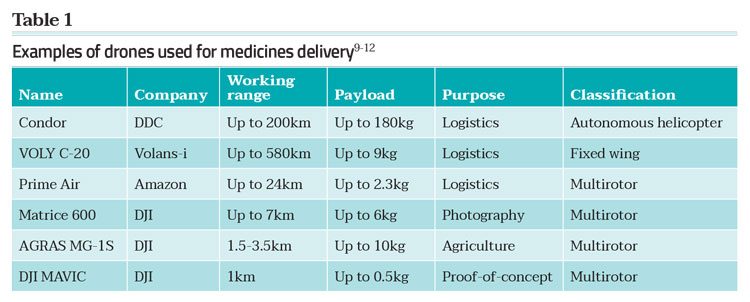

Transport considerations
The two main propulsion designs, ie, rotor – typically classed as vertical take-off and landing (VTOL) – and fixed wing variants, are currently being blurred by the technical advances entering the market. For example, there is a new subset of fixed wing drones, which by a combination of auxiliary rotors can land and take off in a vertical manner but once airborne can fly following the trajectory of a normal fixed-wing aircraft. Other variants involve motors that can tilt either the whole wing of the aircraft, or the rotors, to again allow vertical take off and landing. All designs will impact the stability of medicines transported.
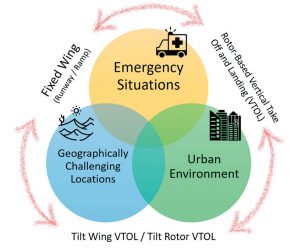

Figure 2: UAV application Venn diagram.
The scenarios in which the drone delivery of medicines will have the greatest impact fall into three main application areas. These are not mutually exclusive and the categories of AUV best suited to these missions can vary depending on the location and type of medicine or medical product to be transported (Figure 2). Speed, cost and the flexibility to traverse physical barriers are the attributes that make drones particularly suitable for the delivery of medicines to emergency situations, within the urban environment and over geographically-challenging locations.
Speed and precision are paramount when transporting life-preserving medicines to emergency situations within the golden hour.13 To perform emergency deliveries when medical professionals are not present at the scene further attributes are important. These include the ability to hover and assess the situation and even the ability to communicate basic instructions before the medicinal products are released. For all scenarios the quality of the medicine must be maintained, and the impact of drone failure on the patient safety must be thoroughly modelled. For example, antivenom injections based on antibodies are sensitive biopharmaceuticals and if the devices used to deliver the medicines are damaged by the drone dropping the medicine close to the emergency, first responders may still attempt to administer the antidote. This may cause additional complications to the patient’s condition if stability of the active molecules has been compromised.


Figure 3: Rule of five to be considered when transporting drones.
The security of the supply chain is important for the drone transport of medicines in the urban environment. Such services represent the final-mile delivery approach, where the medical products will be delivered over short distances from local hubs, which may well be appropriately-sited pharmacies.13 This will reduce pollution, transport time and also open the possibility of the delivery of personalised medicines to patients once prepared at the clinic or hospital pharmacy. However, regular transportation to a particular address may gain the attention of those with the intent of stealing the medicines and diverting their use, thus the delivery packaging must include extra security technologies to protect the contents if the security of the supply chain is challenged. Nevertheless, urban drone delivery could be very important for the transport of chemotherapy in the future, thus keeping vulnerable patients away from potential hospital-borne infections.
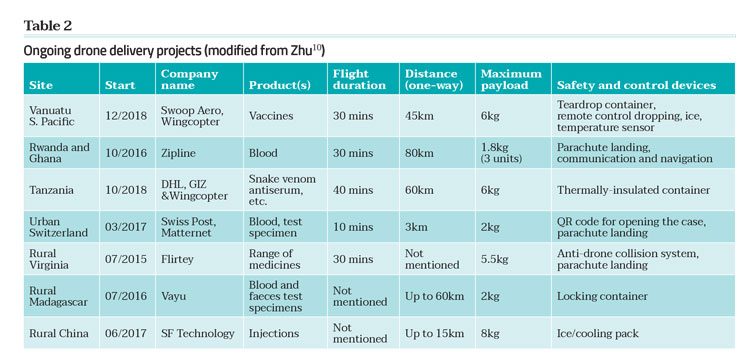
UAV delivery of medicines to geographically-challenging locations must incorporate all the elements described above, which are covered in the rule of five required to maintain the safety and quality of drone-delivered medicines (Figure 3). However, predicting the safe flight time and range, monitoring the on-board conditions and testing the quality of the medicine post flight are even more pertinent. Vaccines, antimalarials and blood products are especially sensitive to spikes in temperature, humidity and vibration. Such products have been successfully flown over many kilometres by the initial developers of drone medical supplies, but there has been little reporting of the impact of drone flight on the quality of these important materials. Typically, when large distances are involved the delivery has been peer-to-peer; ie, medical professional from a medical hub to a field surgery or clinic, providing the potential for assessing the quality of the medicines on arrival by specifically trained staff.10,14 Notwithstanding the concerns of UAV transportation, there has recently been several successful pilot projects using drones to transport medicines and medical products. Snapshots of these are given in Table 2.

Frameworks of the future
As can be seen, there are initiatives underway, with many more under consideration. Commercial packaging solutions that exist are being evolved to meet the specific needs of drone logistics. What is missing, however, is a systematic and open framework for assessing and assuring safety and quality of medical products.
At King’s College London, research has begun on such a framework. Work has started with insulin and continued with vaccines, antimalarials and blood products. At this early stage, five tests that should be applied have been mapped out (proposed) when considering delivering medicines by drone.14 These include:
- Considering the gross weight of the medicines to be transported, the safe flight time and safe range. Testing the edge-of-failure by taking into account the likely variance of environmental conditions is required for the selection of the most appropriate drone
- Carrying out a quality test for the medicine post-delivery to ensure that the pharmaceutical product has arrived in a stable form. Ideally, this should be non-destructive, simple to perform and, if possible, based on pharmacopeia recommendations and QbD considerations such as critical material quality attributes
- On-board monitoring of the medicine’s environment during drone flight is required to record if or when conditions have deviated from the manufacturer’s recommendations. Critical process parameters such as temperature, pressure, vibration frequency and g-force should be monitored
- Ensuring the security of the medicines within the drone supply chain is required; for example, onboard anti-tamper monitoring and recipient authentication
- Understanding the effect of drone failure during flight, considering both the consequences on the medicine and the environment.
Involvement of the various stakeholders, including formulation scientists, packaging developers, logistics experts, regulators and especially pharmacists, will be needed to fully address the new opportunities that medicine delivery by drone offers to both patients and the modern healthcare systems in all regions of the world.
About the authors




References
- Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics-Chemistry, Manufacturing, and Controls Documentation. US 6. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics and Research (CBER). May 1999.
- Jenke D. Accounting for risk when designing chemical 7. safety assessments for pharmaceutical packaging systems. Eur. Pharm. Rev. 2018: 1.
- Xu Q, Ng JSL, Tan OY, Huang Z. Needs and attitudes of Singaporeans towards home service robots: a multi- generational perspective. Uni. Access Inf. Soc. 2015 8. 14 (4) 477-486.
- Sykes C. Time- and Temperature-Controlled Transport: Supply Chain Challenges and Solutions. Pharmacy & 9. Therapeutics.2018;43(3):154–70.
- Amukele T, Ness PM, Tobian AAR, Boyd J, Street J. Drone transportation of blood products. Transfusion. 2017;57(3): 582–588.
- Haidari LA, Brown ST, Feruson M, Bancroft E, Spiker M, Wilcox A. The economic and operational value of using drones to transport vaccines. Vaccine. 2016;34(34): 4062–4067.
- Goodchild A, Toy J. Delivery by drone: An evaluation of unmanned aerial vehicle technology in reducing CO2 emissions in the delivery service industry. Transportation Research Part D: Transport and Environment. 2018;61: 58–67.
- Rimmer A, Iacobucci G, Mahase E. No deal Brexit may worsen drug shortages pharmacists warn. The British Medical Journal 2019 (366) 5226.
- Connolly D. New Rules for New Tools? Exploitative and Productive Lawfare in the Case of Unpiloted Aircraft. Alternatives 2018. 43(3): 137–156.
- Zhu W. The developmental status and challenges of drone use in the transportation of medical supplies (2019) MSc thesis King’s College London.
- Miller PC. Drone Delivery Canada unveils Condor large delivery UAS. Available from: http://www.uasma.com/ articles/1991/drone-delivery-canada-unveils-condor- large-delivery-uas. [Accessed 11 July 2019]
- Lisa E. Amazon Reveals New Details About Drone Deliveries. Available from: https://time.com/4185117/ amazon-prime-air-drone-delivery/. [Accessed 8 July 2019]
- Scott JE, Scott CH. Models for Drone Delivery of Medications and Other Healthcare Items. International Journal of Healthcare Information Systems and Informatics. 2018;13(3): 20–34.
- Hii M, Courtney P, Royall PG. An Evaluation of the Delivery of Medicines Using Drones. Drones.2019;3,52.
Issue
Related topics
Antibodies, Biopharmaceuticals, Drug Safety, Drug Supply Chain, Environmental Monitoring, Packaging, Personalised medicine, QA/QC, Quality by Design (QbD), Regulation & Legislation, Robotics, Supply Chain, Technology, Vaccines









