Trends in pharmaceutical cleanroom technology
Posted: 23 January 2008 | | 1 comment
There are many trends worth reporting in the context of pharmaceutical cleanroom technology: technical as well as regulatory trends. Supporting them is the continuing trend towards worldwide international standards, not only regarding contamination and biocontamination technology, but also regarding related topics such as air filtration. The endeavours for controlling micro-organisms, so competently addressed by the pharmaceutical industry and cleanroom technology, are beginning to spread – and this again is a trend – into further areas of human endeavour such as hospital hygiene.
Isolators versus RABS technology
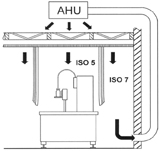

Figure 1: line, protected with conventional cleanroom technology[1]
The predominant trend in aseptic filling technology is the substitution of conventional cleanroom technology by isolators and RABS technology. Both technical concepts are distinguished by offering – if operated correctly – almost a quantum leap of the Sterility Assurance Level.
In conventional cleanroom technology (Figure 1, from Rauschnabel1), the aseptic core is protected with unidirectional downward airflow of HEPA-filtered air. From the surrounding room it is separated by curtains or solid partitions, with air spill-over from the aseptic core into the surrounding environment downstream of the critical area. Operators interfere into the aseptic core with their sterilely gloved hands and sanitisation of the internal surfaces of the processing area is by means of periodical disinfection.
In comparison, the isolator (Figure 2, from 1) offers various additional safety features:
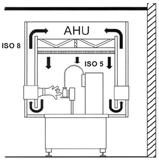

Figure 2: Isolator protected filling line[1]
- strict separation of process and personnel
- isolator-internal air circulation independent from the room air circulation system and, as a consequence, the feasibility of biodecontamination of its internal surfaces by potent agents such as vapour-phase hydrogen peroxide.
Since the late 1990’s, acceptance of the isolator as the most favoured protection scheme for aseptic filling became widespread, and was, for a number of years, almost unopposed. The market census performed by Lysfjord and Porter2,3 at periodic intervals corroborated its continuous advance. In parallel, however, a new technical concept was developed, quite discretely initially: its denomination RABS – Restricted Access Barrier System – entered into the limelight but recently4,5. There are two conceptual approaches: the passive RABS (Figure 3, from 1), where the central HVAC system of the facility is responsible for maintaining the required air quality in the aseptic core, and the active RABS (Figure 4, from 1): a stand-alone unit equipped with its own air handling unit for providing the required conditions in the aseptic core and drawing its supply air from the room surrounding the filling line.
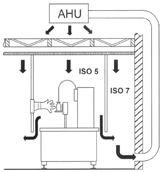

Figure 3: Filling line, protected with a passive RABS system[1]
In both cases, after having provided protection to the aseptic core, the air spills over into its environment – just as in conventional cleanroom technology. Thus, the same room classification for the environment of the filling line is required both for the conventional cleanroom concept as for the two RABS options: room grade B according to the GMP guideline of the European Community6. The isolator, on the other hand, is happy with a room grade C or D environment. Due to the use of the air spill-over principle, RABS is not feasible where combined product and personnel protection is called for. The isolator is the only concept capable of handling this situation safely.
What future trends are to be expected in the competition RABS versus isolator? A most comprehensive assessment of the options available for protecting aseptic filling lines has recently been published by Agalloco, Akers and Madsen7. They considered a total of 22 project criteria, and there were, of course, pros and cons for each of the assessed technologies. Therefore, case by case assessment is indicated for deciding how best to meet a specific protection requirement. However, a clear trend away from conventional cleanroom technology is evident, and this is bluntly highlighted by the authors with their question: Will conventional cleanroom technology still be GMP compatible in 10 years’ time7?
Demise of cleanroom technology? By no means!
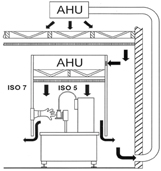

Figure 4: Filling line, protected with an active RABS system[1]
That there seems to be no future for conventional cleanroom technology in aseptic filling does certainly not mean that this is a technology of only historical interest! A most promising future remains, for example, in non-sterile processing of final dosage forms. This is also true for manufacturing the components for sophisticated primary packaging concepts, such as pre-filled syringes and pens, and for the manufacture of diagnostic or investigational devices where the injection-moulding process and associated automatic assembly operations gain prominence. This is indeed a growth market which is adequately addressed with conventional cleanroom technology. However, another trend can also be perceived: a trend towards concepts gleaned from microelectronics. There, for many years system designs based upon Filter-Fan-Units (FFU’s in short) have been very popular and continue to be so. FFU’s are autonomous units consisting of a variable speed fan, sound absorbers and a terminal HEPA filter. Where required, heat exchangers can be installed upstream of the air intake of the unit.
Modern injection-moulding machines are frequently equipped with a FFU above their closure unit, for example, where the product is ejected. In isolators and active RABS units as well, they frequently drive air circulation. But, above all, they permit (Figure 5) extremely simple air circulation concepts widely adopted in semiconductor manufacturing facilities, the so-called wafer fabs. From the processing area, the air is extracted into service chases. It then continues back into the ceiling plenum, where it mixes with the outside air fraction. The FFU’s, interspaced in the ceiling with lighting elements and blind plates, inject the supply air into the production area exactly where the highest contamination risks are located. Air ducts are only required for process air extraction.
Figure 5: Fan-filter-unit (FFU) based air circulation scheme in a semiconductor manufacturing facility. (1 outside air intake; 2 outside air handling unit; 3 mixing plenum chamber; 4 fan filter units; 5 work area; 6 service chases with free return air flow; 7 process air extract duct; 8 process air extract fan – in combination, where required, with an air scrubber for removal of noxious gaseous contaminants; 9 process air exhaust to atmosphere).
This free air recirculation means that the ceiling plenum will automatically be maintained at a slightly negative pressure in comparison with the production area: no false air can therefore enter the processing zones. Process equipment is accessed for maintenance from the service chases where also all equipment connections to gaseous or liquid process media are positioned. Equipment subdivision into a processing and a maintenance area has become widely utilised in microelectronics – will this trend one day also be followed by pharmaceutical filling machinery?
Trends in regulatory guidance
FDA pushes ahead
Preparation of the FDA Guidance for Industry compendium on aseptic processing8 is an excellent example for the benefits of dialogue during the drafting stage. After publication of the first draft as preliminary concept paper – not for implementation9, industry – as exemplified by the comments of the Parenteral Drug Association PDA10 – offered plenty of constructive criticism which led to a second draft for comment purposes – not for implementation11. Again, comments submitted were fairly taken into consideration: comparing the drafts with the final result, it is evident how much the determinations have gained in clarity and precision.
The European position
How about Europe? To start with, a positive statement: the regulatory authority of the European Community – EMEA, the European Agency for the Evaluation of Medicinal Products – acted well ahead of FDA regarding GMP determinations for isolators: a short chapter on that topic was included, for the first time, in the 1998 edition of Annex 1 to the GMP Guide of the European Community6, for example the Annex devoted to manufacturing of sterile medicinal products.
This author regrets having to report that the openness to dialogue distinguishing FDA is not reflected by EMEA. A frustrating example is the continuum of never-ending revisions from which the already mentioned Annex 1 continues to suffer. The latest revision, initiated in September 200512 has still not been concluded.
The topics under revision are:
- room classification
- determinations for process simulations with nutrient media (media fills)
- bioburden determination
- capping of vials
One topic is, regrettably, not addressed:
- determinations regarding unidirectional airflow in grade A areas
Room classification
The EMEA room grades differ from the corresponding FDA determinations in two important aspects:
- determinations have been established for the occupancy states at rest and in operation
- particle concentration limits are set for two particle diameters: 0.5 µm and above as well as 5 µm and above
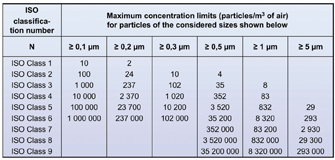

Figure 6: Air cleanliness classification according to ISO 14644-1
The determinations for particles of 5 µm and above are difficult to meet. Before the 2003 edition of Annex 1, a “zero” concentration value applied for these macroparticles for room grade A in the occupancy states at rest and in operation as well as for room grade B in the at rest state. In the 2003 revision, the limit value was reset at 1 particle >_ 5µm per m3 for the above-mentioned room grades, which mathematically was a step in the right direction. What about the practical consequences? Single sample volumes and times are stretched to almost astronomical levels13,14! According to the classification philosophy adopted by the International Standard ISO 14644-115 on cleanroom classification, a total of at least 20 particles should be counted at class limit in order to guarantee a statistically relevant count. Following this philosophy, the single sample volume for the Annex 1 stipulations for particles > 5 µm would be 20 m3 of air! A discrete particle counter with a sampling rate of 1 cu.ft./min = 0.0283 m3/min would require 706 min for taking a single sample! Even with the sequential sampling procedure according to Annex F of ISO 14644-1 the sampling time would still be 136 min provided that not a single particle 5 µm and above was counted during this interval. Such sampling times must be considered as absurd. Even reducing the sampling volume to 1 m3 as suggested in the draft for classification purposes a sampling time of 36 min would result. This is still extremely long for a result without statistical relevance.
Another problem exists when sampling the macroparticles 5 µm and above: the risk of their precipitation in the sampling tube between sampling head and the discrete particle counter (Eaton16). Tubing length should not exceed 1.5 to 2 m so that a considerable number of point-of-use counting units would be required even in a room of modest extension. To make things worse still, the EMEA authorities require validation of the length of tubing and the radii of the bends. How to do that realistically considering the low concentrations of the macroparticles?
The core question is: are the particles 5 µm and above worth all that effort? What meaningful, operationally relevant information is offered by them which is not provided by assessment of the 0.5 µm particles and by the sampling of the airborne micro-organisms? This author therefore pleads that the limit values be set either according to the determinations as given in ISO 14644-1 (Figure 6) or – better still – be abolished altogether.
Air velocity determinations
The topic to be discussed next is not addressed in the revision proposal. Annex 1 continues to require homogeneous laminar airflow in the range of 0.36 – 0.54 m/s in the working area for anything but closed isolators and glove boxes. The maintenance of laminarity should be demonstrated and validated.
There are two things that are wrong with this requirement:
Objection number 1: The terms laminar air flow and laminarity employed in Annex 1 are incorrect language. Laminarity is, correctly speaking, a flow pattern entirely devoid of turbulence. It is inherently unstable at the high Reynolds numbers applying in a clean area of any kind. Therefore, as soon as the airflow enters into contact with equipment surfaces it will automatically transform itself into turbulent airflow. Considering this, the ISO cleanroom standards consistently avoid these misleading terms. Instead, they use the physically correct term, unidirectional airflow without exception.
Objection number 2: The stipulated numerical values. Agreed, a long tradition lies behind such stipulations. However, is there scientific evidence that air velocities in the range 0.36-0.54 m/s always provide the best protection? Instead, a target-oriented determination such as that adopted by FDA should be given preference: to maintain unidirectional airflow of HEPA filtered air at a velocity sufficient to sweep particles away from the filling/closing area and to prove that this requirement is successfully met.
Transparency of decision making
No formal reaction by EMEA to comments and suggestions such as those discussed above has yet been forthcoming. Therefore, it is unknown whether objections raised by industry and professional bodies such as the Parenteral Drug Association PDA17 are being taken into consideration and, if so, to what extent and if not, why not. Judging on earlier experience, there is not much cause for optimism. Most of the comments are by no means new18,19: they have been submitted before, many times repeatedly, without much effect and, as far as this author is aware, without any explanations from the regulatory authorities why they have not been taken into consideration.
The present status of revision
A British MHRA inspector, I. Thrussell, presented a short status report during a recent technical conference20. Accordingly, the ad-hoc task force committed to the Annex 1 revision has terminated its work and submitted the result to EMEA for final approval. The decision of this body was expected to be taken during the month of October 2007. Since then, no further news.
Truly international guidance compendia – a trend to come?
GMP guidelines for manufacturing medicinal final dosage forms continue to be of regional character. Those of Japan, the European Community, FDA and other nations, although reflecting the same philosophy, differ in important detail – as the previous section of this article has shown. Fortunately, a trend towards internationalisation is becoming apparent. One prominent body serving this objective is ICH, the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, established by the regulatory authorities of Japan, the USA and the European Community. The ICH guideline Q7A21 on Good Manufacturing Practice for manufacturing active pharmaceutical ingredients is their first compendium covering a GMP topic. It has since been incorporated, as Part II, into the GMP Guide of the European Community, and has achieved corresponding status in Japan and the USA. May a harmonised GMP guideline on the manufacture of final pharmaceutical dosage forms soon follow.
The ICH Q7A guideline was an excellent first step towards international Harmonisation in the GMP field. However: is ICH the optimum body for such efforts? After all, important pharmaceutical nations remain outside the select ICH group of nations. Therefore, it would be better still if internationality of Harmonisation could be upgraded to ISO level. There is reason for hope: a first precedent already exists. In March 2006 the first ISO standard on a pharmaceutically relevant GMP topic was published: ISO 15378 on GMP requirements for primary packaging materials for pharmaceutical drugs22.
The trend towards science-based risk management in the quality assurance context – pioneered by FDA – has received further backing by the recent publication of the ICH Consensus Guideline Q923. Risk assessment and risk analysis are, after all, powerful tools for identifying the relevant risks of a production scheme, for example, the risks upon which qualification should focus.
Reaching a milestone: the ISO cleanroom standards
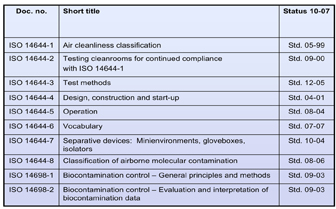

Figure 7: Present development status of international cleanroom standards
Way ahead of the regulatory efforts, the trend towards international Harmonisation had already dominated the scene in cleanroom technology standardisation. Here, a milestone has been reached this year: the first generation of the ISO series of cleanroom technology standards is now complete (Figure 7). Thus, an internationally recognised and accepted bench-mark for quality assurance in contamination and biocontamination control has been created14,24. Regulatory compendia frequently refer to these standards for transforming contamination control postulates into technical solutions.
Following this trend towards international standardisation, drafting of an ISO standard for HEPA and ULPA filters has recently been initiated25.
Beyond pharmaceuticals
Philosophies developed in the context of cleanroom technology and the systematic devotion to quality management distinguishing the pharmaceutical industry and its suppliers enjoy increasing recognition – this is a trend, too – in other spheres of human endeavour. Two examples will be presented.
Microbiological risks receive increased attention in the field of general air-conditioning, both for comfort and for industrial applications. The sick building syndrome and repeated outbreaks of the ‘legionnaires’ disease have prepared the path, and the time has now come for the publication of guidelines stipulating minimum hygienic requirements for HVAC, for example, Heating, Ventilating and Air-Conditioning systems and their components. An internationally recognised landmark here is the guideline VDI 602226 developed by VDI, the Association of German Engineers (Verein Deutscher Ingenieure). VDI guidelines are published bilingually in German and English.
There is another international trend coming up: to finally devote closer attention to nosocomial infections in hospitals. This scientific term may well cause misgivings: what unpleasant happenings are hidden behind that incomprehensible term? Until recently, the transparent term, ‘hospital infection’ or ‘hospitalism’ was the commonly used expression for this class of disease. For hospital pharmacists, above all, it is frustrating to observe how the meticulous orientation towards product safety which distinguishes both industry and the pharmacist ends at the pharmacy’s door. At this point, this author may be crossing the border-line towards polemics, but this is done without qualms of conscience, as he is in good company. The editor of the prestigious European Journal of Parenteral and Pharmaceutical Sciences recently gave the following title to an editorial27: Massacre of the innocents. Its message was to denounce – spiced with inimitable British humour – the lamentable state of control regarding nosocomial infections so apparent in many hospitals.
The discrepancy between the highly developed quality management systems distinguishing the pharmaceutical industry and their suppliers of cleanroom technology and the situation apparent in many hospitals is indeed quite shocking. May a trend result from this: to spread the impact of uncompromising quality thinking into many new areas of human endeavour – in the hospital and elsewhere.
References
- Rauschnabel J.: Zwischen Isolator und Sterilraum (Between isolator and sterile room) – Restricted Access Barrier System (RABS). Pharm. Ind. (Pharmaceutical Industry) 68 (2): 767-773 (2006).
- Lysfjord J., Porter M.: Barrier isolation history and trends. Pharmaceutical Engineering 23 (2): 58-64 (2003).
- Lysfjord J., Porter M.: Barrier isolation history and trends, a millennium update. Pharmaceutical Engineering 21 (2): 142-148 (2001).
- Lysfjord J.: The ISPE RABS definition: an introduction. Pharmaceutical Engineering 25 (6): 116-120 (2005).
- Isberg E.A.: RABS and isolator operations. Pharmaceutical Engineering 27 (1): 18-21 (2007).
- Eudralex, the rules governing medicinal products in the European Union – Vol. 4: EU Guide to Good Manufacturing Practice for medicinal products for human and veterinary use. European Commission, Brussels (December 2005).
- Agalloco J., Akers J., Madsen R.: Choosing technologies for aseptic filling: “Back to the future, forward to the past?” Pharmaceutical Engineering 27 (1): 8-16 (2007).
- Guidance for industry: Sterile drug products produced by aseptic processing – current Good Manufacturing Practice. U.S. Department of Health and Human Services, Food and Drug Administration, Rockville MD/USA (September 2004).
- Sterile drug products produced by aseptic processing – draft. U.S. Department of Health and Human Services, Food and Drug Administration FDA, Rockville MD/USA (preliminary concept paper – not for implementation, 27. September 2002).
- Madsen R.: PDA comments on the FDA preliminary concept paper “Sterile drug products produced by aseptic processing”. PDA Letter 38 (11): 1, 11-15 (2002).
- Guidance for industry: Sterile drug products produced by aseptic processing – current Good Manufacturing Practice. U.S. Department of Health and Human Services, Food and Drug Administration, Rockville MD/USA (draft guidance for comment purposes only – not for implementation, 22 August 2003).
- GMP Annex 1: Proposals for amendment to the environmental classification table for particles and associated text, amendment to section 42 concerning acceptance criteria for media simulations, amendment to section 52 concerning bioburden monitor-ing, and additional guidance in section 88 on the sealing of vials. European Medicines Agency, Inspections, London (21 September 2005).
- Schicht H.H.: The revised Annex 1 to the EC GMP guideline. GMP Review 2 (3): 7-11 (2003).
- Neiger J.: Cleanroom standards. European Pharmaceutical Review 12 (2): 92-96 (2007).
- ISO 14644-1: Cleanrooms and associated controlled environments – Part 1: Classification of air cleanliness. International Organization for Stardardization ISO, Geneva (May 1999).
- Eaton T.: Annex 1 of the EC Guide to Good Manufacturing Practice (EC cGMP) and continuous particle monitoring – help or hindrance for cleanroom manufacturing? European Journal of Parenteral & Pharmaceutical Sciences 12 (2): 29-37 (2007).
- Roessling G.: PDA comments on proposed changes to EU GMP Annex 1. PDA Letter 42 (6): 29-31 (2006).
- Lingnau J.: Requirements on the manufacture of sterile medicinal products according to EEC-guide. Swiss Contamination Control 3 (4b): 103-105 (1990).
- Gail L. et al.: Revised Annex 1 of the EC GMP guide: VFA comments and interpretation. European Journal of Parenteral Sciences 4 (4): 127-129 (1999).
- Verbal communication by I. Thrussell, Medicines and Healthcare Products Regulatory Agency MHRA, London, during the ISPE Conference on Barrier Isolation Technology, Berlin, 18 September 2007.
- ICH Harmonised Tripartite Guideline ICH Q7A: Good Manufacturing Practice for active pharmaceutical ingredients. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH, Geneva (November 2000; incorporated as Part II into the GMP guideline of the European Community).
- ISO 15378: Primary packaging materials for medicinal products – Particular requirements for the application of ISO 9001:2000 with reference to Good Manufacturing Practice (GMP). International Organisation for Standardisation ISO, Geneva (March 2006).
- ICH Q9 Consensus Guideline: Quality risk management. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH, Geneva (November 2005).
- Schicht H.H.: International cleanroom standards: a tool for compliance. Eur. Pharm. Rev. 10 (3): 98-102 (2005).
- Hogan H.: Filter industry is standardizing, hopefully, on a standard. Clean-Rooms 20 (5): 7-8 (2006).
- German guideline VDI 6022: Hygiene- Anforderung an Raumlufttechnische Anlagen und Geräte – Blatt 1 (Hygienic standards for ventilation and airconditioning systems and components – Part 1). Beuth Verlag, Berlin (April 2006).
- Sharp J.: Massacre of the innocents. European Journal for Parenteral & Pharmaceutical Sciences 11 (4): 95 (2006). Hans H. Schicht Dr. sc. Techn, Dr. Hans Schicht Ltd Dr Hans H. Schicht is an independent consultant in cleanroom and contamination control technology with more than 30 years practical experience in this field. As the official Swiss representative, he was actively engaged in the international standardisation efforts in this field for many years.
Hans H. Schicht
Dr. sc. Techn, Dr. Hans Schicht Ltd
Dr Hans H. Schicht is an independent consultant in cleanroom and contamination control technology with more than 30 years practical experience in this field. As the official Swiss representative, he was actively engaged in the international standardisation efforts in this field for many years.









Thanks I like this article.