Product Quality Lifecycle Implementation (PQLI) – providing practical solutions
Posted: 23 January 2008 | Bruce Davis (Chairman of ISPE’s International Board of Directors) | No comments yet
This article discusses ISPE’s Product Quality Lifecycle Implementation (PQLI) initiative, which is to provide practical guidance for implementation of ICH Q8, Q9 and Q10. It represents the author’s individual opinion. It should be noted that PQLI is an evolving work area and so will continue to develop beyond the position explained at the time of writing this article.
Background and ICH
Historically, the industry has grown from operating on a national basis to develop and manufacture medicines that are now available globally. In today’s climate, for a pharmaceutical company to be as efficient as possible it is key that the interpretation of regulations, guidelines by both industry and regulators is as harmonised as practically possible.
This international aspect has meant that good local practices may become more widely applicable and perhaps be universally applied. Engineering companies with worldwide operations for example, may act as a catalyst to spread best practices in plant design, as learning can be applied from building a plant in one country to designing another project in another country. The same principles may be applied to pharmaceutical product and process development and manufacturing.
The guidelines written by the International Conference on Harmonisation (ICH) are of particular significance. Although these are not mandatory, they do provide wide reach, as ICH is a unique forum made up of both industry associations and regulatory organisations from the United States, Europe and Japan. Additionally, ICH guidelines may be adopted by other regulatory agencies – for example ICH Q7A has effectively become a global standard for API (Drug Substance) GMPs.
A family of guidelines is under development within ICH. The following guidances have recently been developed:
- ICH Q8 Pharmaceutical Development (completed – in implementation phase)
- ICH Q8R (Annex to Pharmaceutical Development Q8(R) – at step 3 of the ICH process )
- ICH Q9 Quality Risk Management (completed –i n implementation phase)
- ICH Q10 Quality Pharmaceutical System (at step 3)
These three together can be considered as capturing many of the principles of how pharmaceutical products may be developed and manufactured in future. They recognise the importance of a science- and risk-based approach. They are, however, generally high level in format and therefore require interpretation in practice.
Within ICH an Implementation Working Group (IWG) is being set up in 2008 to drive and communicate consistent interpretations of ICH Q8/9/10 implementation.
One of the challenges has been to convert the interpretation of regulations and guidances into practical outputs. Questions arise, such as the following:, What are the implications in practice of Quality by Design (QbD)? How does Process Analytical Technology (PAT) apply to my manufacturing processes?
In terms of the relationship between Quality by Design and Process Analytical Technology, the view of the author is that PAT techniques provide supporting tools to enable product and process understanding.
ISPE’s Product Quality Lifecycle Implementation Initiative
ISPE’s Product Quality Lifecycle Implementation (PQLI) has been developed to provide practical and pragmatic implementation of ICH Q8/Q9/Q10. The initiative is intended to help interpret the new three ICH guidances appropriately and provide practical and useful detail. It therefore also provides supporting information and technical processes in relation to QbD.
PQLI is a global industry-led initiative, which has a plan over about five or more years, as shown in Figure 1.
In order to produce practical guidances, it is envisaged that four steps are necessary:
- To stimulate debate on key subject areas. These informal discussions are cross-industry and regulators are invited to attend. It will debate topic areas where the principles may be clear but finding practical outputs to deliver such principles is less clear. It will use case studies and real examples to illustrate problems and proposed solutions.
- To capture, in written form, the outcomes from the debates and then to obtain comments. These would be via ‘white papers’ or articles published in journals, such as ISPE’s peer-reviewed Journal of Pharmaceutical Innovation.
- To produce guidances.
- To carry out training.
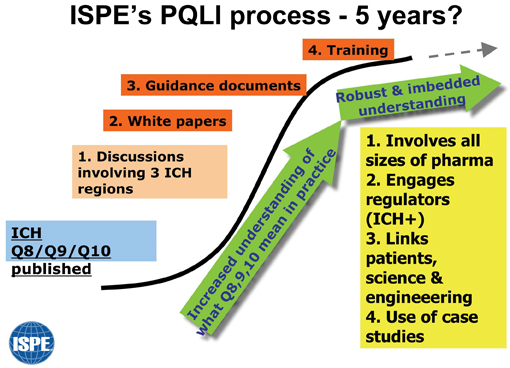

Figure 1: Diagram to show PQLI plan over next few years
Step 1 has already started. A launch session was held at the PQLI conference in Arlington, Virginia, USA, on 5-6 June 2007. The intentions at this conference were to provide an open forum to discuss topics where confusion or apparent hurdles exist and to look for common and pragmatic understanding in these areas. Case studies were used to stimulate discussion and participants contributed to the enthusiastic and informal debates.
A subsequent discussion took place in Berlin in September 2007.
For the future, a conference is taking place in Copenhagen 9-11 April 2008 and will be a major forum for discussions from a European perspective. Steps are also being taken to invite Japanese and US input to ensure all three ICH regions will be represented.
Publication of white papers following these various discussions, are expected during 2008.
Topics for discussion and development
Three key topics were selected at Arlington for discussion:
- Criticality
- Design Space
- Control Strategy
The reason for these being chosen is that there has been much debate within industry to try to clarify these areas. At Berlin an additional topic was added: - Legacy Products
Task Teams have been formed to address each of the four topics above. The teams are international in composition and made up of representatives from large and small companies.
Below is some of the initial thinking from the first three task teams, applicable at the time of writing this article. The fourth task team has only just started work. The information will change as the teams continue to refine and develop the details.
Criticality (Task team 1)
Evaluation of criticality would normally be focused on areas where scientific knowledge and judgment indicate a likely effect on product safety and efficacy.
The level and rigour of evaluation may vary depending upon the level of risk associated with a particular factor. A primary objective of the Task Team was to define a strategy to assign categories of criticality for variables associated with the manufacture of a pharmaceutical product. Ultimately, sponsors could apply that strategy to characterise and maintain pharmaceutical quality across the product lifecycle. In addition, the process to categorise criticality is intended to be applicable to API and drug product manufacturing processes.
ICH Q(8)R provides useful definitions for Critical Quality Attribute and Critical Process Parameter:
- Critical Quality Attribute (CQA): A physical, chemical, biological or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality.
- Critical Process Parameter (CPP): A process parameter whose variability has an impact on a critical quality attribute and therefore should be monitored or controlled to ensure the process produces the desired quality.
While the focus of ICH Q(8)R Annex to Q8 Pharmaceutical Development guideline is on drug product, these definitions and the proposed PQLI criticality decision tree in Figure 2 provides a useful guideline for categorising criticality for API as well.
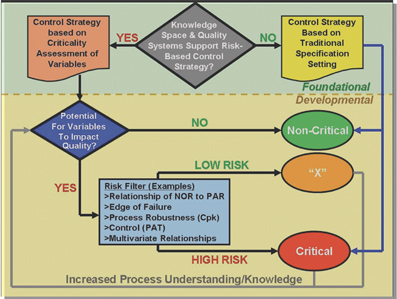

Figure 2: PQLI Criticality Decision Tree to Delineate Categories and Levels of Criticality Across the Product Lifecycle (NOR = Normal Operating Range PAR = Proven Acceptable Range CpK = Measure of process robustness PAT = Process Analytical Technology)
Within the pharmaceutical industry, categorisation between Critical and Non-Critical variables has multiple implications depending on context. Many pharmaceutical companies differentiate criticality for business criteria (for example operating efficiency) separately from criticality used to delineate attributes and parameters for pharmaceutical quality purposes.
While a universal definition for the word “critical” may be desirable to categorise any variable during Criticality Analysis, a well defined, robust and non-prescriptive risk assessment process is fundamental and should enable relevant and justifiable categories of criticality from which to judge pharmaceutical quality.
A process to delineate Critical from Non-Critical variables has been drafted for discussion and is illustrated in Figure 2.
Design Space (Task team 2)
The ICH Q8 definition for design space is “the multidimensional combination and interaction of input variables (for example, material attributes) and process parameters that have been demonstrated to provide assurance of quality.”
Design Space is dynamic and begins at drug conceptualisation and continues to evolve over the entire lifecycle of the process. At the time of product initial commercialisation, the design space can be considered to represent the best overall process understanding at the time. It continues to evolve, not because of lack of development effort, but as additional knowledge and information is generated throughout the lifecycle of the product – a key facet of continuous improvement programmes. As such, it would normally require change management under a company’s quality and change control systems. It is expected that changes proposed to the design space would require further regulatory interactions, but changes within the design space may be managed via the quality system.
Design space and its links to overall product and process knowledge and normal operating ranges (NOR) are represented in Figure 3.
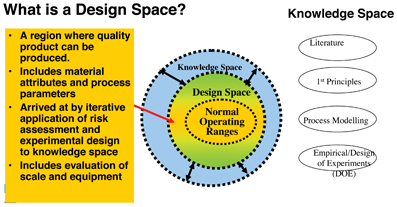

Figure 3: PQLI Diagrammatic representation of Design Space
Design space is determined during product development by an iterative process. The process is not complete until successive iterations demonstrate appropriate understanding of attributes needed to assure the target product profile. The procedure starts with an understanding of the target product profile. While this may vary with a specific product, for many pharmaceutical presentations, the profile needed will be widely understood. Understanding of clinical needs and therapeutic index are important, for example, to determine this profile. Initially, the links of the process to meet the profile may be tenuous, as central elements of safety margins, clinical dose range, and the like become understood in parallel with early conceptualisation and development of the process.
Control strategy (Task team 3)
Control Strategy is defined in the ICH Q10 (Step 2) document as:
“A planned set of controls, derived from current product and process understanding that assures process performance and product quality. The controls can include parameters and attributes related to drug substance and drug product materials and components, facility and equipment operating conditions, in-process controls, finished product specifications, and the associated methods and frequency of monitoring and control.”
ICH Q8 and particularly Q8(R) (Step 2) also give information about the principles of Control Strategy, for example in section 2.5 within the ‘Elements of Pharmaceutical Development’, and Section 3.3 within ‘Submission of Pharmaceutical Development and Related Information in Common Technical Document (CTD) format.’
This Control Strategy for a particular product should be established within a framework comprising the Pharmaceutical Quality System (See ICH Q10) including GMP. In ICH Q8(R) (Step 2) document the ‘Minimal Approach’ to Control Strategy is contrasted with the ‘Enhanced, Quality by Design Approach.’
In the former, drug product quality is primarily controlled by intermediate and end-product testing. In the ‘Enhanced Approach’ drug product quality is ensured by a risk-based control strategy for well-understood products and processes, and quality controls are shifted upstream in the manufacturing process to enable the possibility of real-time release or reduced end-product testing. When a product has been developed using the ‘Enhanced Approach’ and a design space has been established, then the control strategy should ensure that the product is manufactured within the design space.
The Product Quality Lifecycle Implementation control strategy model
The task team has proposed a PQLI Control Strategy Model to facilitate communication and understanding of the concept and provide a framework for a structured approach to the development and implementation of a control strategy. (Figure 4). The Model seeks to link the attributes of the product that are important to the patient, to the controls in the manufacturing process that are needed to deliver those attributes, and also to show in parallel the business requirements.
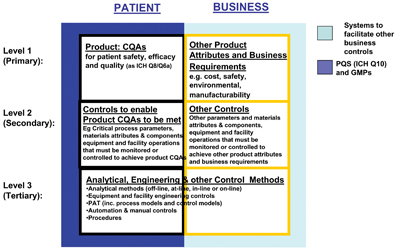

Figure 4: the PQLI Control Strategy Model
The Model was initially proposed at the Arlington conference in June 2007 and gained positive acceptance from attendees.
he Model is split horizontally and vertically to distinguish between:
- What is critical to the patient vs. what is critical to the business – two columns
- How manufacturing and engineering controls link to patient and business requirements – three rows or levels.
The Model is presented in Figure 4 and is still a draft under discussion.
The Model highlights areas of importance, for example, related to patient safety, efficacy, and quality and proposes information needed for effective science and risk based technical processes. Note that this is a framework for discussion and each product must be treated on a case by case basis.
Also note that product specific patient-related Quality Attributes need to work within a Pharmaceutical Quality System (PQS), as per ICH Q10 (shown in Figure 3 by the dark blue background to the Patient side), whereas non-pharmaceutical product aspects may be governed by other business systems, such as operator safety, meeting environments needs, cost effective manufacture (shown in Figure 3 by the light blue background to the Business side).
Summary
From the initial response, PQLI has been well received. Its approach to provide practical and pragmatic solutions seems to be something the industry wants. Although PQLI does not produce regulatory documents, it is believed that the technical processes it will produce should act as a positive enabler for discussions with regulators to explain the details behind a science and risk based approach.
Acknowledgements
As co-chair of the Control Strategy team, I have very much valued the tremendous help and support from many people – especially the Control Strategy team members, the other task teams and the Steering team. It is not possible to list everyone here, but I would like to particularly express thanks to:
- ISPE PQLI Steering Team (Chairs – Russ Somma, Ron Branning, David Selby)
- PQLI task team chairs:
Criticality – Tom Schultz/Roger Nosal
Design Space – Jim Spavins/John Lepore
Control Strategy co-chair – Line Lundsberg Legacy Products – Chris Potter
Bruce Davis
Chairman ISPE’s International Board of Directors
Bruce Davis is the current Chairman of ISPE’s International Board of Directors. He is one of the leaders of their PQLI (Product Quality Lifecycle Implementation) initiative, and leads the task team on Control Strategy. He has given many presentations and been involved in educational events for ISPE in America, Europe and Asia-Pacific. Regarding other organisations, he also is secretary to ASTM E55.03 on Pharmaceutical Standards.
He is a professional engineer, has wide international knowledge and has been involved in projects in many countries, for example for sterile operations, oral solid dosage, packaging, bulk plants and laboratories. He has run workshops on Quality by Design approaches for new products. Bruce works for AstraZeneca.
Issue
Related topics
ICH guidelines, Process Analytical Technologies (PAT), Product Quality Lifecycle Implementation (PQLI), Quality by Design (QbD)









