Platform technology for routine three dimensional cell culture
Posted: 19 June 2008 | | No comments yet
Cell culture assays play an important role during the first stages of pharmaceutical development. The design of such in vitro models is significant and data resulting from such tests directly influences the progression of compound development. Therefore it is becoming progressively more important to design cell culture assays that are more representative of the behaviour of cells in living tissues. As a consequence, investigators are developing technology to enhance the cell culture environment and enable cells to grow in ways resembling their in vivo counterparts. This is particularly relevant to the design of culture models that enable three dimensional cell growth in vitro.
The growth of mammalian cells in the tissue culture laboratory provides an important resource for researchers developing new pharmacological reagents. Cell cultures are frequently used as the first step to assess the effect of compounds on living tissues. Our industry is continually looking for new enabling technology that will improve the process of drug discovery, including the development of more accurate in vitro assays. In addition, there is a progressive move toward reducing the numbers of animals used in research and the culture of cells is expected to relieve some of this pressure. The market for cell culture technology is enormous and is expected to rise as a billion dollar industry for years to come1. Accordingly, there is a drive to develop new cell culture technologies, including media formulations, growth supplements and specialised cell culture plastic ware.
Standard Petri dishes, culture flasks or multi-welled culture plates provide a substrate for cell growth in vitro. However, the growth of cells on these planar two-dimensional (2D) surfaces does not compare to the complexities of the three-dimensional (3D) environment cells experience when they are a component of tissues in the body. Cells grown on 2D plastic adapt their shape to the flat surface and re-model their cytoskeleton. In addition, the opportunity for cultured cells to interact with their neighbours is limited and most often occurs, particularly in confluent cultures, in a restricted area on their periphery. These changes to the cell’s geometry, the spatial environment it occupies, and its interaction with adjacent cells and the extra-cellular matrix, impacts on its ability to function in a representative manner. This is of concern to the researcher attempting to evaluate the effectiveness and toxicity of candidate drugs since the outcome of such experiments influence whether further development and investment in the research programme is justified. To reduce the risks involved in taking forward the development of a compound, it is important to validate its function in models that most closely represent living tissues. Consequently, various enabling technologies have been developed to create more favourable environments for cell growth in vitro, including systems that enable 3D cell culture.
Demand for technology to enable 3D cell culture
The maintenance of cell viability and function outside the body is challenging. It must be acknowledged that many important discoveries have resulted from the discovery of cells grown in conventional 2D systems. However, the 2D surface upon which cells grow in the Petri dish does not compare to the complex geometries of the environment that cells experience in living tissues. The context in which a cell grows matters and changing its surroundings can significantly modify its behaviour. Evidence clearly shows that the characteristics of cells cultured as multi-cellular 3D masses significantly alters their function compared to their growth as standard 2D cultures2. These and many other findings conclude that superior cell function can be attained when cells adopt more realistic 3D geometries and form complex spatial relationships with adjacent cells. Consequently, there is demand for new cell culture technology that enables 3D cell growth to more authentically mimic the in vivo environment.
In vitro models that enable routine 3D cell growth will be of significant scientific and commercial value. In terms of basic research, such systems will provide more representative models to study the molecular mechanisms that regulate cell behaviour. For applications in the pharmaceutical industry, it is acknowledged that modification of cell culture growth conditions to those that more closely imitate the in vivo environment is a necessary step to developing more accurate predictive models of drug activity3. Moreover, reports indicate that the response of cells to chemical reagents is significantly influenced by their growth in 3D culture systems4. It follows that the design and development of new in vitro models that enable 3D cell growth will find important applications in the drug discovery business.
Substantial interest in materials science and the design of specialised cell culture apparatus has developed in response to this demand. Multi-disciplinary approaches involving chemists, engineers and biologists have generated a series of new technologies to satisfy the demand for a culture technology that enables 3D cell growth.
Current trends in 3D cell culture
With the advent of tissue engineering and the development of regenerative therapies, there has been a drive to create technology to enable 3D cell growth for subsequent clinical transplantation of tissues created in the laboratory. Much attention has been focused on the fabrication of specialised materials and scaffolds to provide structure and support to the developing tissues, many of which are biodegradable and are subject to rigorous evaluation prior to clinical use. Whilst considerably less energy has been directed toward developing similar technologies for exclusive use in cell culture applications, a number of alternative strategies have been developed. Notably: the employment of hydrogels containing materials such as agarose, collagen or fibrin provide a 3D environment for various cell types5; biodegradable polymers such as poly(glycolic acid), poly(lactic acid) and their copolymers poly(lactic-co-glycolic acid) for use in vitro6; the fabrication of synthetic substrates consisting of electrospun fibres that provide a 3D topography on a micron scale to enhance growth and functional of cultured cells7; the development of alginate scaffolds for growth of cell aggregates8.
Many of these technologies have been developed with specific applications in mind although no one approach satisfies the demands of a generic platform technology for in vitro use only. Whilst biodegradability of the scaffold may be important for the transplantation of in vitro derived tissues, it is not a preferred feature of matrices employed for supporting 3D cell growth in vitro. An inert scaffold that does not change its structure or composition over time would be favoured since it would help maintain a stable environment and reduce variability. Scaffold thickness is also an important feature, especially in the absence of a circulatory system, where cultured cells rely on the exchange of gases, nutrients and waste products with the growth medium. Diffusion over long distances and the establishment of unstirred layers results in inefficient exchange to the detriment of cell viability and optimal function. Several of the commercially available technologies for 3D cell growth do not take this factor into account and scaffolds can be several millimetres thick. This also poses a problem of getting cells into and out of the material itself. The use of hydrogels in tissue engineering and cell culture applications is recognised, but the general everyday use of such gels in vitro is restricted by various practical issues including gel storage, preparation, variability and expense. The type of material that is chosen to serve as a support to enable 3D cell growth is important since users should ideally determine whether their cell preparations react differently to the material compared to their standard growth medium (which is most likely to be 2D polystyrene culture ware).
Finally, it is important that the scaffold is of the appropriate dimensions to offer an optimal environment for 3D cell growth. Whilst cells grow differently on electrospun fibres, this technology does not enable 3D cell growth since the fibres merely create a micro-topographical surface upon which cells grow and are therefore still considered 2D in nature. The opposite also applies in that if the dimensions of the scaffold are too great, cells just grow on the 2D surfaces within structure of the scaffold itself and are unable to form true 3D interactions with neighbouring cells.
Platform technology to enable routine 3D cell culture
The majority of current cell culture assays rely on the growth of established cell lines and primary cells on 2D polystyrene surfaces. Culture ware manufactured from polystyrene may therefore be considered as an industrial standard for in vitro cell growth and users are familiar its inert features and characteristics. Confidence in this growth substrate has been maintained in a new platform technology for routine 3D cell culture whereby the configuration of the polystyrene substrate has been re-engineered as a highly porous scaffold. Such materials are manufactured using a process known as emulsion templating to produce porous matrices consisting of polymerised styrene monomers and cross-linking molecules suitable for cell culture applications (Figure 1 on page 50)9.
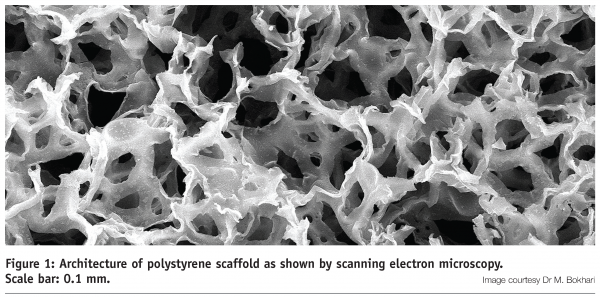

This method of production enables tight control over the porosity of the polystyrene scaffold to within narrow tolerances10 and can be partly automated for scaled manufacture11. This is an important feature to create a robust uniform structure which is consistent and suitable environment for 3D cell growth. The scaffold is subsequently engineered into thin membranes (0.2 mm thick) and adapted to existing cell culture plastic ware (Figure 2). The thickness of the membrane is an important feature of this technology since it enables the entry of cells into the interior structure of the scaffold (and out again for cell retrieval) and allows sufficient exchange of gases and nutrients with cells growing within it. Like traditional polystyrene culture ware, the scaffold can be irradiated and supplied pre-fabricated, sterile and ready to use. Such features offer several advantages to the user including an inexpensive, simple un-wrap and use consumable technology for routine 3D cell culture.
Validation of this technology has been performed on a broad variety of different cultured cell types. The polystyrene scaffold provides sufficient vertical space to enable cells to grow, differentiate and function in 3D9,12,13 (Figure 3).
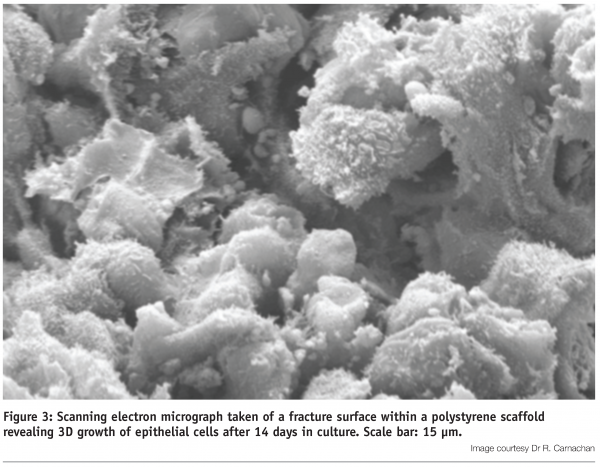

Under optimal conditions, cells form layers and develop complex 3D interactions with their neighbours, ultimately occupying all the space within the lattice (Figure 4). Experiments show that cell viability is maintained at high levels during 3D culture and cells are not exposed to the un-natural geometric stresses experienced by their counterparts cultured on 2D plastic surfaces. Indeed, cells grown in 3D culture respond to biochemical agents in a manner resembling the activity of tissues in vivo13.
Conclusion
The expense of developing new pharmaceuticals is escalating and the cost associated with drugs failing at advanced stages of development is high. To minimise the risks of taking compounds forward in development, it is essential to test and evaluate their function in well validated and accurate in vitro assays. The introduction of a platform technology that enables routine 3D cell culture will provide biotechnologists with an opportunity to grow cells and tissues in the laboratory that are more representative of their native counterparts. Such models will play an important role in improving the efficiency of the drug discovery process.
References
- Cell/tissue Culture Supplies: Global Strategic Business Report. Global Industry Analysts Inc., 2006.
- k. Schmeichel, M. Bissell: Modelling tissue-specific signalling and organ function in three dimensions. J Cell Sci, 116, pp. 2377-88, 2003.
- K. Bhadriraju, C. Chen: Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov Today, 7, pp. 612-20, 2002.
- T. Sun et al: Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol, 122, pp. 372-81, 2006.
- S. Blackshaw: Promotion of regeneration and axon growth following injury in an invertebrate nervous system by the use of three-dimensional collagen gels. Proc Biol Sci, 264, pp. 657-61, 1997.
- A. Mikos et al: Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials 14, pp. 323-30, 1993.
- M. Schindler et al : Living in three dimensions: 3D nanostructured environments for cell culture and regenerative medicine. Cell Biochem Biophys, 45, pp. 215-27, 2006.
- M. Dvir-Ginzberg : Ultrastructural and functional investigations of adult hepatocyte spheroids during in vitro cultivation. Tissue Eng, 10, pp. 1806-17, 2004.
- M. Bokhari et al: Novel cell culture device enabling three-dimensional cell growth and improved cell function. Biochem Biophys Res Commun 354, pp. 1095-100, 2007.
- R. Carnachan et al: Tailoring the morphology of emulsion-templated porous polymers. Soft Matter, 2, pp. 608-616, 2006.
- M. Bokhari et al: Effect of synthesis parameters on emulsion-templated porous polymer formation and evaluation for 3D cell culture scaffolds. J Mat Chem, 17, pp. 4088-4094, 2007.
- M. Hayman et al: Enhanced neurite outgrowth by human neurons grown on solid three-dimensional scaffolds. Biochem Biophys Res Commun, 314, pp. 483-88, 2004.
- M. Bokhari et al: Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J Anatomy, 211, pp. 567-76, 2007.