The importance of disaccharide excipients in biologics
Posted: 17 February 2021 | Bastiaan Dickhoff (DFE Pharma), Sunil Kumar Nataraj (DFE Pharma), Thontesh GC (Apotex Pharmaceuticals) | No comments yet
Many biologic formulations use a variety of high-purity injectable grade disaccharide excipients to ensure functionality and stability of the final product. In this article, Sunil Kumar Nataraj, Bastiaan Dickhoff and Thontesh GC explore the use of these sugars in the stabilisation of biologics and provide recommendations to manufacturers for enhancing supply chain security.


The essential role excipients play in biologic drugs, alongside the gaps in supply chain security that have been exposed by drug shortages related to the COVID-19 pandemic, is alarming.9 In addition, recent quality issues that have resulted in the closure of plants, heightened trade tensions and the growing numbers of biologics that require low-temperature storage and shipment, place an even greater strain on (bio)pharmaceutical supply chain systems.9
This review explores the current knowledge of stabiliser excipients and offers general recommendations on excipient and formulation parameters to be applied in biologics. In addition, we provide recommendations for enhancing supply chain security of the high-purity disaccharide excipients that are extensively used in the lyophilisation of biologics. The latter is of particular importance given the current market situation of original BLA and biosimilar applicants relying on a single supply source for their excipients. It is recommended that such companies work with reliable partners who supply excipients in alignment with International Pharmaceutical Excipients Council (IPEC) guidelines.
Disaccharide excipients in lyophilisation
The type and quality of excipients plays a vital role in the development and optimisation of lyophilisation process parameters. The use of biologic drug-specific excipients contributes significantly to the drug solution stability (bulk phase). It also aids in the subsequent optimisation of lyophilisation cycles, with faster drying conditions to achieve the desired lyophilised cake appearance, along with other critical quality attributes (CQA) such as optimal pH, moisture content and reconstitution time.
An ideal excipient for lyophilisation should be chemically stable, inert, safe, multifunctional and economical.2 Excipients should provide a stable product with an acceptable lyophilised cake structure, low reconstitution time, specific pH and low residual water content. An improperly formed lyophilised cake, such as a ‘meltback’ or a ‘collapse’, will lead to incomplete reconstitution and unacceptable product quality. Low residual water content is needed to ensure stability and prevent loss of activity of the biologic. Low reconstitution time is also necessary for the biologic to be administered quickly to the patient. In most cases, following lyophilisation the finished product is reconstituted in water for injection/saline/five percent w/w dextrose and injected either via the intravenous (IV) or subcutaneous (SC) injection route.
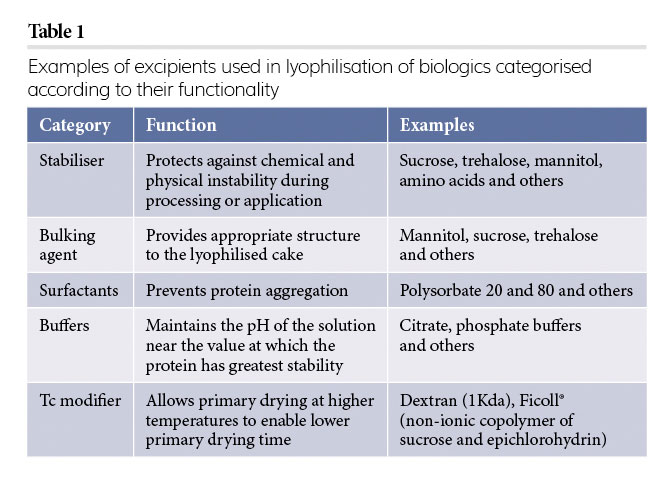

Various excipients are used in lyophilisation to achieve several objectives. Table 1 summarises the most important applications of excipients that are used in the lyophilisation process.
Lyophilisation involves freezing the product (ice formation), primary drying (sublimation) and secondary drying (desorption), which exposes the biologic drug to freezing and drying stresses. An annealing step is often introduced during freezing to ensure efficient primary drying. High‑purity disaccharide excipients, such as sucrose and trehalose, protect the biologic drugs from the freeze-related (cryoprotectant) and drying-related (lyoprotectant) stresses. There are two theories for the stabilisation of biologics by disaccharides: the vitrification theory (kinetic stability) and the water replacement theory (thermodynamic stability).3 The vitrification theory refers to immobilisation of biologics in a matrix of an amorphous disaccharide, while the water replacement theory refers to the formation of hydrogen bonds between the hydroxyl group of the disaccharide and the polar groups of the biologic drug.
Key parameters to be studied during freeze drying for an appropriate stability and shelf life of a biologic formulation are: 1) the glass transition temperature (Tg) of disaccharide excipients and Tg of the combination of the in situ formed amorphous (glassy) disaccharide-protein (biologic drug); 2) the Tg of the maximally freeze concentrated solution (Tg’); and 3) the collapse temperature (Tc), beyond which the lyophilised cake collapses. Any temperature increase beyond Tc would result in a meltback (collapse).4
Disaccharides with a high Tg are said to be beneficial for maintaining biologic drug stability.3 The biologic drug is expected to increase the Tg of the formulation; therefore, disaccharides with a high Tg are known to complement the formulation.6 However, all of this depends on the type of biologic drug studied.
Primary drying should be carried out below the Tc otherwise there will be a loss of lyophilisate structure and its biological activity. However, many excipients have a low Tc threshold. To achieve efficient primary drying with reduced drying time, a Tc modifier is often used which helps dry at higher temperatures during primary drying. Furthermore, to reduce the primary drying time, the ratio of stabiliser to bulking agent must be optimised (with a starting ratio of 1:2 of stabiliser to bulking agent).3
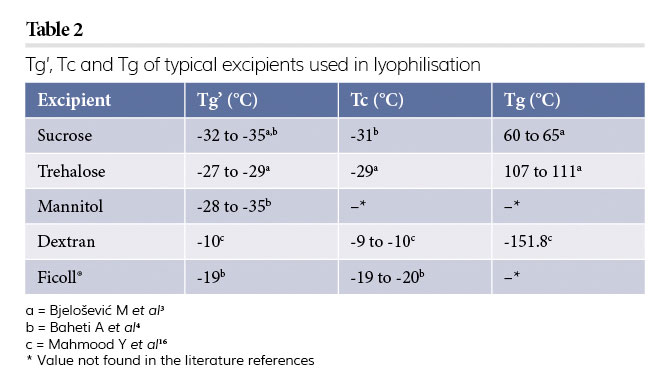
A summary of stabilisers, Tc modifiers, along with critical temperatures are shown in Table 2.

Aside from temperature control during the lyophilisation process, the avoidance of the Maillard reaction via the use of certain reducing disaccharides plays an important role in the formulation of stable biologic drugs. The degradation rate due to the Maillard reaction generally increases with increasing moisture content, with little or no degradation below a water activity of 0.25 and with a maximum degradation around 0.75. Because of this, non‑reducing disaccharides, such as sucrose and trehalose, are mainly used in biologic drugs.3 Sometimes lactose monohydrate is used; for instance, in the stabilisation of glucarpidase.5
The primary drying phase is the most time- and energy-consuming stage in the lyophilisation process and significantly contributes to the total cost of lyophilisation.3 Secondary drying involves the removal of the last traces of water by desorption. In this process, product temperature would be less than or about 50°C and drying time should not be greater than six hours to prevent loss of activity of the protein (biologic). In most cases, target moisture content of the finished product (cake) is less than one percent w/w. This can be assessed by Karl Fischer water content analysis.3
Another important factor contributing to the stabilisation of biologics is the ratio of stabiliser to biologic drug used in the formulation. For effective stabilisation, a general recommendation is that the molar ratio should be at least 360:1 for stabiliser to biologic drug to avoid denaturation.3
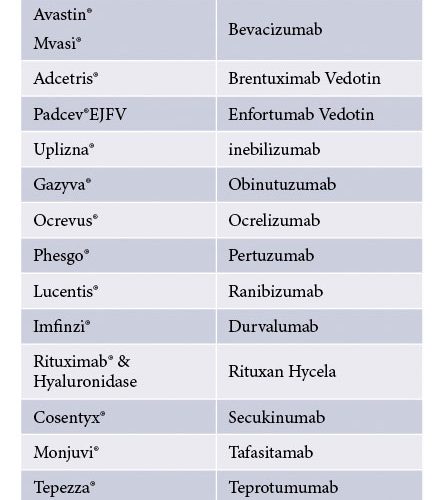

Table 3: Some marketed formulations containing trehalose7
The use of stabiliser excipients with high Tg’, Tc and Tg is beneficial for reduction of primary drying. For this reason, trehalose is increasingly being used in mAb formulations as the first choice excipient5 (Table 3). Selection of the right stabiliser excipient depends on the type and concentration of the biologic drug together with the physicochemical compatibility with other excipients in the formulation. It is important that the stabiliser excipient is in an amorphous state throughout the shelf life of the product (lyophilised cake). There are few reported cases of cake instability that are associated with crystallisation of mannitol when used as a stabiliser. In contrast, both sucrose and trehalose are not known to crystallise during the lyophilisation process and therefore are often more effective in the stabilisation of the biologic drug than mannitol.3
Current usage of disaccharide excipients
The disaccharide excipients sucrose and trehalose have been used in several commercialised biologic drug products, as described in Tables 3 and 4. To support pharmaceutical companies in the manufacture of biologic drugs (including vaccines), DFE Pharma has extended its portfolio with high purity low endotoxin-grade trehalose and sucrose under the BioHale® brand.12
Supply chain security of disaccharide excipients
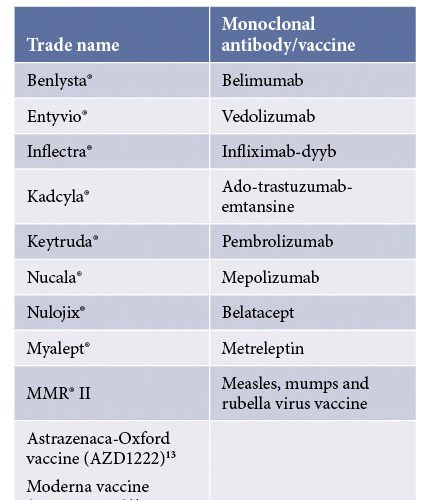

Table 4: Some marketed formulations containing sucrose as a stabiliser
The need to improve the pharmaceutical supply chain was recognised even before the COVID-19 pandemic.9 Several events in recent history have put pharmaceutical supply chains under pressure and as such have demanded supply chain improvements. These events include, among others: quality issues at manufacturing sites, closure of plants for environmental reasons, heightened trade tensions and the rising demand in emerging markets in Asia-Pacific/Latin America. The growing numbers of biologics that require low-temperature storage shipment has also influenced drug shortages in recent years.9
The current COVID-19 pandemic has added further pressures to the security of pharmaceutical supply chains. A survey held by Pharmaceutical Technology (April 2020) on the confidence of the pharma supply chain in maintaining adequate supply of medicines during the outbreak of COVID-19, showed that 42.2 percent of the respondents felt “doubtful”.9 This shows the importance, for all companies involved, of establishing proactive strategies for building and maintaining supply chain security.
Sourcing excipients through a global supply chain network will likely be essential for supporting globalised pharma manufacturing. Companies that supply excipients to pharmaceutical companies internationally need to have multiple supply points spread across the globe to supply their materials within the requested delivery times. In addition, excipient suppliers should have a strengthened and secured supply chain system, in which they have good control over end-to-end processes, from sourcing their raw materials to delivery of their excipients to the manufacturers of finished drug products.
The IPEC Guide and various IPEC presentations10,11 on the qualification of excipients outlines various topics that excipient suppliers and pharmaceutical companies could and should work closely together on in order to enhance supply chain security. For instance, supplier qualification is an important part of managing and securing the supply chain for a pharmaceutical company. In addition, pharmaceutical companies are recommended to utilise supplier questionnaires, review pharmacopoeial compliance, audit their suppliers’ facilities and identify any risks involved in excipient parameters beyond those mentioned on COA.


Sunil is a pharmaceutical leader with on-the ground experience of providing problem-solving solutions across the US, Europe, Japan and India. Sunil joined the Technology & Innovation department at DFE Pharma in 2017 as product application specialist in Germany. Sunil has pharmaceutical product development expertise in oral solids focusing on ODT’s and continuous twin screw granulation supporting continuous manufacturing. Sunil focuses on enhanced customer R&D interaction catering to current pharma market trends and ever changing pharma regulatory landscapes.


Bastiaan leads the Product Application Specialists team at DFE Pharma and his expertise lies in understanding powder behaviour in batch and continuous processes coupled with multivariate analysis and quality by design. Prior to working at DFE Pharma, he worked at RSSL and GlaxoSmithKline. Bastiaan holds a PhD from the Department of Pharmaceutical Technology and Biopharmacy at Groningen University, the Netherlands, where he specialised in the relationship between the role of lactose (excipient) and APIs for inhalation purposes. He has published various papers on the relationship between lactose and APIs.


Thontesh is currently Manager within the Global R&D group at Apotex Pharmaceuticals. He has 14 years of experience in sterile formulation design, process development, scale-up and process validations of injectables, micelles, pre-filled systems, controlled drug delivery systems and ophthalmic solutions along with product lifecycle management. Thontesh is a subject matter expert in lyophilisation cycle designs, development and optimisation. This also includes working with different types excipients, such as various sugars, polymers, buffers and solvents used for lyophilised product development.
References
1. Vaccines, Blood & Biologics [Internet]. U.S. Food and Drug Administration. 2021 [cited 21 January 2021]. Available from: https://www.fda.gov/vaccines-blood‑biologics.
2. Rayaprolu BM, Strawser JJ, Anyarambhatla G. (2018) Excipients in parenteral formulations: selection considerations and effective utilization with small molecules and biologics. Drug Dev. Ind. Pharm. 44, 1565–1571.
3. Bjelošević M, Pobirk AZ, Planinšek O, Grabnar PA. (2020) Excipients in freeze-dried biopharmaceuticals: Contributions toward formulation stability and Lyophilisation cycle optimisation. Int. J. Pharm 576 119029.
4. Baheti A, Kumar L, Bansalb A. (2010) Excipients used in lyophilization of small molecules J. Excipients and Food Chem. 1 (1).
5. Mensink MA, Frijlink HW, Maarschalk KVDV, Hinrichs WLJ. (2017) How disaccharides protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions Eur. J. Pharm. Biopharm, 114 288–295.
6. Bjelošević M, Seljak KB, Trstenjak U, Logar M, Brus B, Grabnar PA. (2018) Aggressive conditions during primary drying as a contemporary approach to optimise freeze-drying cycles of biopharmaceuticals. Eur. J. Pharm. Sci Off. J. Eur. Fed.Pharm. Sci. 122, 292–302.
7. DailyMed [Internet]. Dailymed.nlm.nih.gov. 2021 [cited 21 January 2021]. Available from: https://dailymed.nlm.nih.gov/dailymed/index.cfm.
8. Excipients information for Pfizer-BioNTech COVID-19 Vaccine [Internet]. SPS – Specialist Pharmacy Service. 2021 [cited 20 January 2021]. Available from: https://www.sps.nhs.uk/articles/excipients-information-for-pfizer-biontech-
covid-19-vaccine/.
9. David Alvaro P. Rethinking the Global Pharmaceutical Supply Chain Post-COVID-19 [Internet]. Pharmasalmanac.com. 2021 [cited 20 January 2021]. Available from: https://www.pharmasalmanac.com/articles/rethinking-the-global-pharmaceutical-supply-chain-post-covid-19.
10. Zawislak P. How to manage risk in your Excipient supply chain. IPEC presentation. Aug 2019.
11. Kraemer E, Lekebusch A. Supply Chain Security and Good Distribution Practice: Essential for Pharmaceutical Excipient fest Europe. Aug 2014.
12. DFE Pharma launches ultimate stabilization aid for biologics [Internet]. Dfepharma.com. 2021 [cited 20 January 2021]. Available from: https://www.dfepharma.com/Corporate/News-and-Events/
News/DFE-Pharma-launches-ultimate-stabilization-aid-for-biologics.
13. COVID-19 A. AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19 [Internet]. Astrazeneca.com. 2021 [cited 20 January 2021]. Available from: https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html.
14. COVID-19 A. AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19 [Internet]. Astrazeneca.com. 2021 [cited 20 January 2021]. Available from: https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html.
15. Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study | Pfizer [Internet]. Pfizer.com. 2021 [cited 20 January 2021]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against#:~:text=(NYSE%3A%20PFE)%20and%20BioNTech,on%20November%208%2C%202020%20by
16. Mahmood Y, Farooq U. Excipients Use in Parenteral and Lyophilized Formulation Development.
Open Science Journal of Pharmacy and Pharmacology. Vol. 3, No. 3, 2015, 19-27.
Issue
Related topics
Biologics, Bioprocessing, Drug Supply Chain, Excipients, Freeze Drying, Lyophilisation, Manufacturing, Supply Chain