Successful Process Analytical Technology (PAT) implementation in pharmaceutical manufacturing
Posted: 29 September 2008 | | 1 comment
The use of Process Analytical Technology (PAT) while a relatively new concept to the Pharmaceutical Industry has been a tried and tested concept in the petrochemical industry for many years. The adaptation of PAT systems by the Pharmaceutical Industry was accelerated by the recent initiatives of the regulatory authorities globally to modernise the Pharmaceutical Industry and focus on enhancing product quality. The common theme of the various initiatives is “planning for quality,” (i.e.) building quality into the products as compared to the traditional paradigm of testing the product to ensure quality.
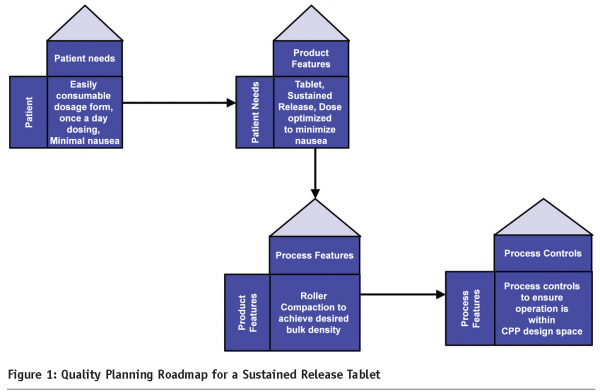
The Juran trilogy concept identifies quality planning, quality control and quality improvement as three fundamental aspects of quality planning1. Quality planning is the process of identifying the needs of the customer and designing the product and the process to meet the needs of the customer. Figure 1 provides an example of a quality planning roadmap for a sustained release product. This map serves as the foundation for the development of the product/process and is the primary requisite for Quality by Design (QbD).

PAT in development
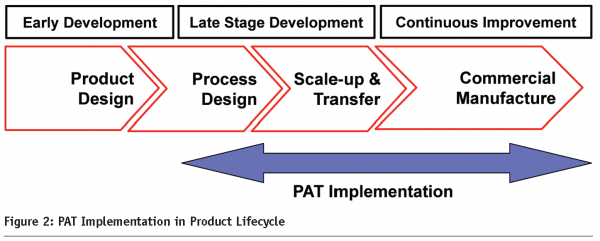
PAT is often associated with in-process control testing in commercial manufacturing. However, in order to successfully implement PAT, sufficient knowledge of the process/product has to be gained in the early stages of development. As part of a QbD effort, PAT opportunities can be identified in early development. However, since the success rates for compounds in early development (Phase 1) is low, it is recommended that PAT application be initiated in the later phases of development (phase 2 and continued into Phase 3). This approach is depicted in Figure 2, for a standard solid dosage form.

A key concept in the application of PAT analyzers as part of the drug product control strategy is that not all attributes that can be measured, need to be controlled. A risk based decision taking the process/product understanding into consideration should be utilized to select the PAT sensors as part of the control strategy.
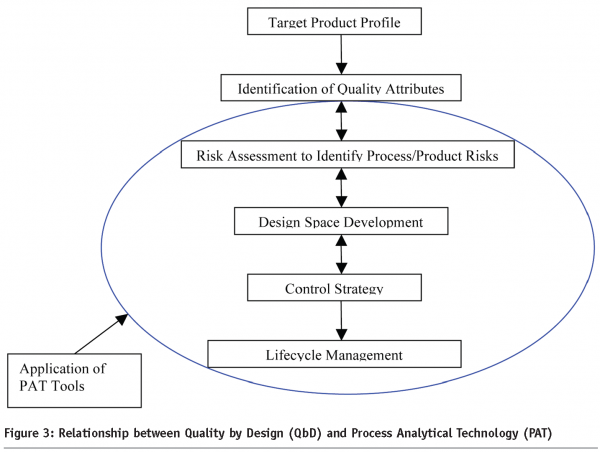

Figure 3 depicts the relationship between QbD and PAT. PAT is one of the many tools that facilitates enhanced process/product understanding and eventually forms the basis of the control strategy in manufacturing. Real Time Release (RTR) on the other hand is an outcome of the enhanced process/product knowledge gained during the development of the product. In Figure 3, the Target Product Profile (for Quality)2,3,4 is the quality roadmap for the Pharmaceutical Scientist and forms the basis for product/process development. It enables the identification of quality attributes that may be important to the product/process and facilitates the use of Quality Risk Management4 to identify areas of high risk. Processing steps/attributes that may need to be monitored to increase process knowledge and mitigate risk are identified and evaluated utilizing appropriate process analyzers to enhance process understanding. Figure 4 demonstrates the output of a quality risk assessment (QRA) exercise for an immediate release solid dose product using a wet granulation technique.
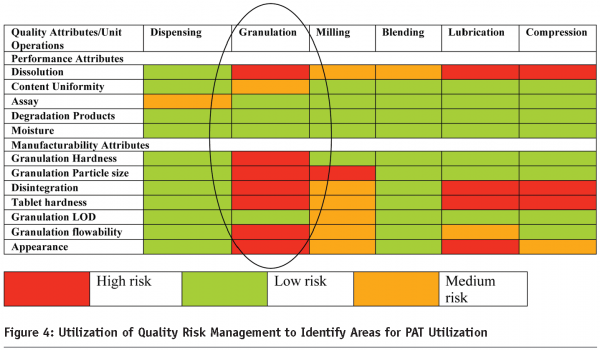

In the case of this product, the granulation unit operations was identified as the area of the highest risk and PAT sensors will be used to monitor the attributes of the granulation and enhance the process knowledge of this unit operation. In development, it is typical to investigate the feasibility of multiple process analyzers to study an attribute (or a process parameter). Identifying and utilizing analyzers is just one aspect of a PAT system. The FDA guidance identifies PAT as a system that comprises four distinct tools which are:
- Multivariate tools for design, data acquisition, and analysis
- Process analyzers
- Process control tools
- Continuous improvement and knowledge management tools.
As process analyzers are being applied either on-line, at-line or in-line to gain process knowledge, it is equally important to focus on the other tools of the PAT system. Multivariate data analysis systems when used in an appropriate manner help identify complex interactions that may typically not be noticed in univariate studies. In combination with the knowledge gained during development and appropriate design and construction of the process equipment, the analyzer enables the definition of process signatures. These signatures can be used for process monitoring, control, and endpoint determination in instances where such signatures may impact product quality. A simple example of such a unit operation would be the monitoring of a blending unit operation using a spectroscopic technique. In this instance the endpoint for blending will be based on the process signature of the quality attribute (uniformity of the blend) as compared to the traditional method of using an endpoint based on time.
Once risk-based decisions are made to identify the appropriate analyzers to monitor product/process variables, it is necessary to ensure that the methods are fit for purpose. This includes an appropriate design of studies to understand the impact of variations to the methods (both from an analytical measurement and a multivariate methodology perspective). Typical variations that need to be considered include variation of inputs (API, raw material) – physical and chemical variations, personnel variations, environmental variations, instrument variations etc. In the case where critical product attributes have an existing reference method, a comparison of the reference method to that of the PAT method must be completed. This will further demonstrate that the PAT system is fit for use and is a suitable alternative to measure the critical quality attribute(s) of the product. In addition to the variations, sufficient understanding regarding the placement of the sensor in the manufacturing equipment and the ability of the device to measure a representative sample should also be demonstrated. Additionally, the development of a scientific and a statistically sound risk based sampling plan should also be one of the goals during development. It is very important to utilize the knowledge gained during atypical manufacturing runs during the development of PAT as they provide a very good opportunity to assess the capabilities of the system.
As one proceeds to the later phases of development (Phase 3 etc) and product/process knowledge has resulted in the development of a design space, it is very important to understand the impact of the PAT systems throughout the design space. This will assist in the development of an adequate control strategy (see Figure 3). The control strategy for a product should be holistic and should be developed based on the impact of the changing characteristics of the product (e.g. powder blend to granulation to tablet) across the entire manufacturing process.
PAT in commercial manufacturing
When designing a control strategy, a useful technique is to focus on the first three PAT tools (see above) and to apply them individually to evaluate each manufacturing step. Multivariate tools should be considered both as a way to capture development knowledge about process variables and also as a way to structure experiments to gain necessary knowledge (e.g. designed experiments). This knowledge supports the definition of the design space, which can be considered as a combined description of the variables and their associated ranges over which quality can be assured. The ISPE-PQLI initiative has posted a Control Strategy position paper6 (for comments) that defines strategies based on either product performance (impacting the safety or efficacy of the drug product) or product manufacturability (impacting business aspects such as yield).
Process controls, (the third tool in the PAT system) are an integral part of the control strategy and are designed based on process knowledge (gained from multivariate tools) and process information (gained from analyzers). There are different levels of process control. One level is the proper design and construction of manufacturing equipment so that it is suitable for its intended purpose and can achieve the required quality. Another level is feedback control, where information from an analyzer is used to adjust process parameters and product quality attributes. A PAT system for a single unit operation may contain components of both levels.
These tools (multivariate design/analysis, and process controls), should be applied together with process analyzers as a system. The advantage of considering each tool separately when creating the system is that it facilitates good decision making. However, each tool influences the others. Risk-based techniques provide a powerful means to link the tools together in a predictable fashion and assist with assigning priorities of effort during experimentation. Risk analysis also provides a means to compare different scenarios, such as one type of control tool versus another.
The fourth PAT tool, continuous improvement and knowledge management, is also linked with the other three but it can appear later in the design of a control strategy. It becomes extremely important in the life cycle of manufacturing quality assurance systems. As with the other three tools, it is useful to consider continuous improvement methods individually by manufacturing step. Essentially, a pro-active program should be established to continually challenge process understanding and to evaluate the effectiveness of the process analyzers and process controls. Good continuous improvement tools should provide a predictive ability to see potential gaps in quality controls, even when all quality criteria are being met.
Continuous improvement also means that constant evaluation of potential quality assurance improvement opportunities should be made. It may be appropriate to conclude that, if the quality assurance system includes some amount of finished product testing, then opportunities are available to improve upon the PAT tools that are in place. In other words, if finished product testing is routinely necessary as part of the information required to make disposition decisions, then the goals of PAT have not yet been fully realized.
Quality systems in a commercial environment
The implementation of PAT in commercial manufacturing will require an alignment of the quality systems with ICH Q-107 to ensure the science and risk based approach to quality is engrained into the operational aspects of the manufacturing site. This includes proactive reactions to failures in the PAT systems, effective change control systems to manage movement within the design space, Systems for the maintenance and update of chemometric models, handling of atypical results in the PAT process etc. Pro-active contingencies for the various failure modes will enable the efficient resolution and reaction to the various situations that may be seen during the implementation of PAT systems in commercial manufacturing. This may include the availability of backup measurement systems that can be used in the event of the primary unit being rendered non-functional, development of sampling plans that may be used for an investigation in when atypical results are observed, or for release testing using reference methods in the case of a failure of all the PAT systems. It is important to understand that these proactive measures should be risk based and may need to be revised as further process/product understanding is gained. An example of one such measure has been provided in Figure 5.
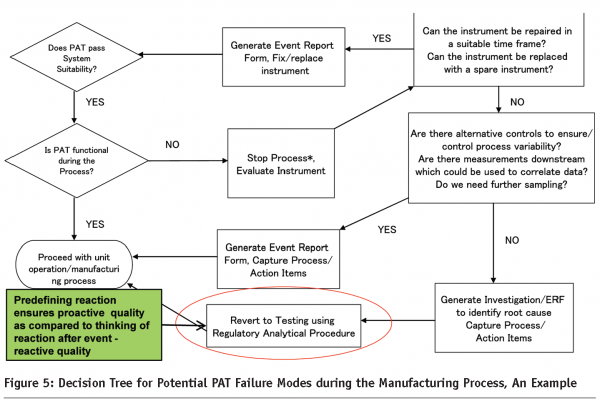

In the event that the sponsor plans to implement a Real Time Release Approach, then the criticality of a robust risk and science based quality systems is even more significant.
The future of PAT
In the pharmaceutical manufacturing plant of the future, ICH Q 8, 9, 10 (and 11) will no longer be guidelines or optional approaches to development and quality. Instead, following the ICH guidance’s will be the expectation of regulatory agencies globally. Companies will have to adopt scientific and risk-based approaches to development, manufacturing and quality. The resulting Quality by Design and PAT control strategies will play an ever-increasing role in ensuring robust and efficient manufacturing processes, delivering high quality products in a cost-effective manner. In the future, PAT systems will be integral parts of development and manufacturing equipment, with standardized interfaces enabling the collection of huge amounts of data processed by IT systems networked in a global knowledge management system. Such an approach will enable the development of high level product and process knowledge even during relatively early phases of drug development, and culminate in manufacturing processes with feed-forward and feedback loops compensating for inherent variability in input materials resulting in finished products assured of meeting specifications – the basis for real time release (RTR) quality systems and internal post-approval change management systems. The QbD processes and procedures needed by engineers, analytical chemists, formulators and quality professionals will be described through technical white papers, regulatory guidance documents, ASTM E55 standards, etc., facilitating a consistent, global approach to QbD development and manufacturing of pharmaceutical products.
That is the vision for the future. However, today there is much uncertainty both from industry and regulators on how to apply QbD concepts during development for small as well as large molecules. While the high-level principles are easily grasped, the devil is in the details, and those details will take time to work through. The reality today suggests we have a long way to go to reach the vision. However, there is progress. Global harmonization is slowly moving forward. Interpretation of ICH guidelines is underway in ISPE, AAPS, PDA and other organizations. ICH itself is forming an expert working group to help define the way forward. Colleges and universities are developing the workforce of the future necessary to ensure QbD/PAT principles become the norm, not the exception in development and manufacturing. These scientists will be skilled in risk management, chemometrics, process analyzers, and pharmaceutical engineering in addition to the “typical” skill sets of analytical chemistry, industrial and physical pharmacy, math and statistics. One thing is clear QbD concepts are slowly gaining traction within the pharmaceutical industry.
References
- J. M. Juran, “Juran on Quality by Design – The New Steps for Planning Quality into Goods and Services,” 1992, Free Press.
- ICH Q8(R) – Pharmaceutical Development Annex to Q8, step 2 version, November 2007, ICH harmonized tripartite guidelines. In International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.
- Pharmaceutical Quality by Design: Product and Process Development, Understanding, and Control, Lawrence X.Yu, Pharmaceutical Research, 2007.
- Pharmaceutical Development Case Study – “Ace Tablets”, 15-16, CMC IM Working Group case study, www.conformia.com.
- ICH Q9 – Quality Risk Management, step 4, November 2005, ICH harmonized tripartite guidelines. In International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.
- PQLI Control Strategy Model and Concepts, Bruce Davis, Line Lundsberg and Graham Cook, Journal of Pharmaceutical Innovations, 2008 (3), 95-104.
- ICH Q10 – Pharmaceutical Quality System, Step 4, 2008, ICH harmonized tripartite guidelines. In International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.
Issue
Related topics
ICH guidelines, Process Analytical Technologies (PAT), Product Quality Lifecycle Implementation (PQLI), Quality by Design (QbD)
Related organisations
Related people
John Levins, Stephen P. Simmons, Thirunellai G. Venkateshwaran










Thank’s for effective information.