Developing broad-spectrum vaccines that target the heart of mutating viruses
Posted: 3 August 2022 | Alexandre Le Vert (Osivax) | 1 comment
Alexandre Le Vert, CEO and Co-Founder of Osivax, explores some evolving approaches to vaccine development that aim to counter the effects of virus mutation.

The COVID-19 pandemic has demonstrated the need for an improved vaccine approach to temper the serious threat of constantly mutating viruses, such as influenza and coronaviruses, on global health. Current vaccines often employ a B-cell immune response through the production of specific antibodies that target the virus’ surface, which can be highly prone to mutation. This strategy cannot anticipate virus mutations and leaves individuals vulnerable to infection when new strains emerge. New T cell-based approaches move beyond the surface and can be combined with B-cell vaccines to address the internal, less variable, parts of viruses to provide broad-spectrum protection against current strains and those yet to come.1
Addressing the ever-changing virus
The surface of a virus is covered with exposed proteins or antigens, which enable it to bind to the receptors of a host cell. Once attached, the virus can alter the function of a cell, allowing it to replicate and produce thousands of copies of itself. Eventually, this leads to the breakdown of the cell’s membrane, releasing more viruses into circulation that can proceed to infect nearby cells. Viruses such as influenza or Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2; the cause of COVID-19) typically infect cells located in the respiratory system, causing inflammation of the trachea and/or the lungs.
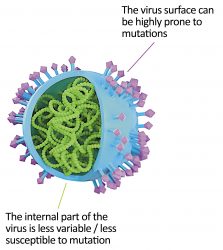

Figure 1: Internal and external virus structures2
In influenza and SARS-CoV-2, the haemagglutinin and spike surface antigens are capable of spontaneous mutation and thus display several functioning shapes of these antigens, further splitting themselves into various strains or variants. As a virus circulates and infects host cells to replicate and proliferate, this new virus entity could carry a different protein structure than its ancestral virus, leading to the emergence of a new strain. These spontaneous mutation events are quite common and often do not lead to an improved version of the pathogen. However, sometimes, the new variant exhibits advantageous features, such as faster propagation due to an improved affinity of its mutated surface antigens with the host cell receptors.
Current vaccine strategies and their limitations
Alongside improved sanitation and better nutrition, vaccination remains one of the most cost-effective measures to reduce the burden of infectious diseases. Most currently available vaccines are developed to treat viruses by attacking surface antigens. In influenza, the haemagglutinin is targeted using a small dose of the weakened virus to trigger the production of specific antibodies that bind to it, thereby neutralising the virus when a real infection arises. These quadrivalent influenza vaccines (QIV), however, lack the capacity to produce cross-reactive responses that can neutralise multiple influenza strains. Cross-reactivity is a necessary component in targeting the antigenic plasticity of the haemagglutinin. Each year, global vaccine organisations must ‘predict’ the future circulating strain months in advance. If the prediction is incorrect, this could lead to antigenically drifted viruses and potential mismatches with vaccine strains.3 Improved, broad-spectrum vaccines are needed to address the challenges posed by the difficulty and time-sensitivity of making accurate predictions against these highly mutating viruses.
The COVID-19 pandemic has demonstrated the need for an improved vaccine approach to temper the serious threat of constantly mutating viruses”
One approach to achieving this goal is to target the more conserved regions of the virus, such as internal antigens, which are not under selection pressure from the antibody component of the immune system and are therefore far less prone to mutations. Unlike surface antigens that are visible on the membrane of the virus and can be detected by antibodies, internal antigens are inside the virus and can only be detected by the cellular T-cell component of the immune system.
The adaptive immune response to a natural infection
Our immune system creates new cells daily to combat harmful pathogens. When a virus escapes the first line of defence (the innate immune response) and starts replicating widely, the immune system deploys a highly specific adaptive response composed of two arms: antibody responses that can directly neutralise the virus, and cytotoxic T cells that will destroy and clear the cells infected by the virus.
When a cell is infected by a virus, all the proteins produced by the infected cell, including its inner component, are presented on the cell surface. Cytotoxic T cells can recognise those viral proteins and release chemical signals to induce apoptosis, or the death of the infected cell. These cells also produce memory T cells to respond faster and prevent future infections from occurring. Presenting surface antigens of a virus to the immune system to trigger an antibody-based response has been the landmark of vaccine development. It has worked efficiently against the dangerous pathogens that cause measles, rubella and tetanus. However, relying solely on this vaccine strategy to tackle viruses with high mutation rates has its limitations, as mutating surface antigens evolve constantly. Several biotechs in the space have identified that vaccines that combine a T cell-mediated immune response with the antibody response could potentially improve protection in line with the natural function of the immune system.
Improved, broad-spectrum vaccines are needed to address the challenges posed by the difficulty and time-sensitivity of making accurate predictions against these highly mutating viruses”
Both B-cell and T-cell responses are mediated by two different synergistic pathways that can recognise different parts of the virus and attack at different stages of its proliferation. Several approaches are in development, including nanoparticles or messenger RNA (mRNA) vaccines that have been engineered to display multiple regions of the haemagglutinin known to provide better cross-protection across all lineages of a single virus.
Another disruptive approach, explored by private biotech companies such as Osivax or Imutex, consists of vaccines targeting the heart of a virus, rather than its mutating surface, and doing so with the cellular component of the immune system (T cells).
Targeting the heart of the virus with a T-cell approach
Osivax, a French biotech, has developed its proprietary oligoDOM® technology platform to elicit a T-cell immune response targeting the internal antigens of a virus. Its mechanism of action relies on the combination of (i) a cargo effect of nanoparticles presenting seven copies of the targeted antigens and (ii) a targeting and activating sequence towards the immune system’s cellular surfaces. Results demonstrate the oligoDOM® platform’s ability to trigger a universal response capable of protection against all variants of a single virus. In influenza, the company’s lead programme, OVX836, targets the nucleoprotein – an inner and conserved antigen, which has more than 90 percent genetic similarity among A-strains of the influenza virus.
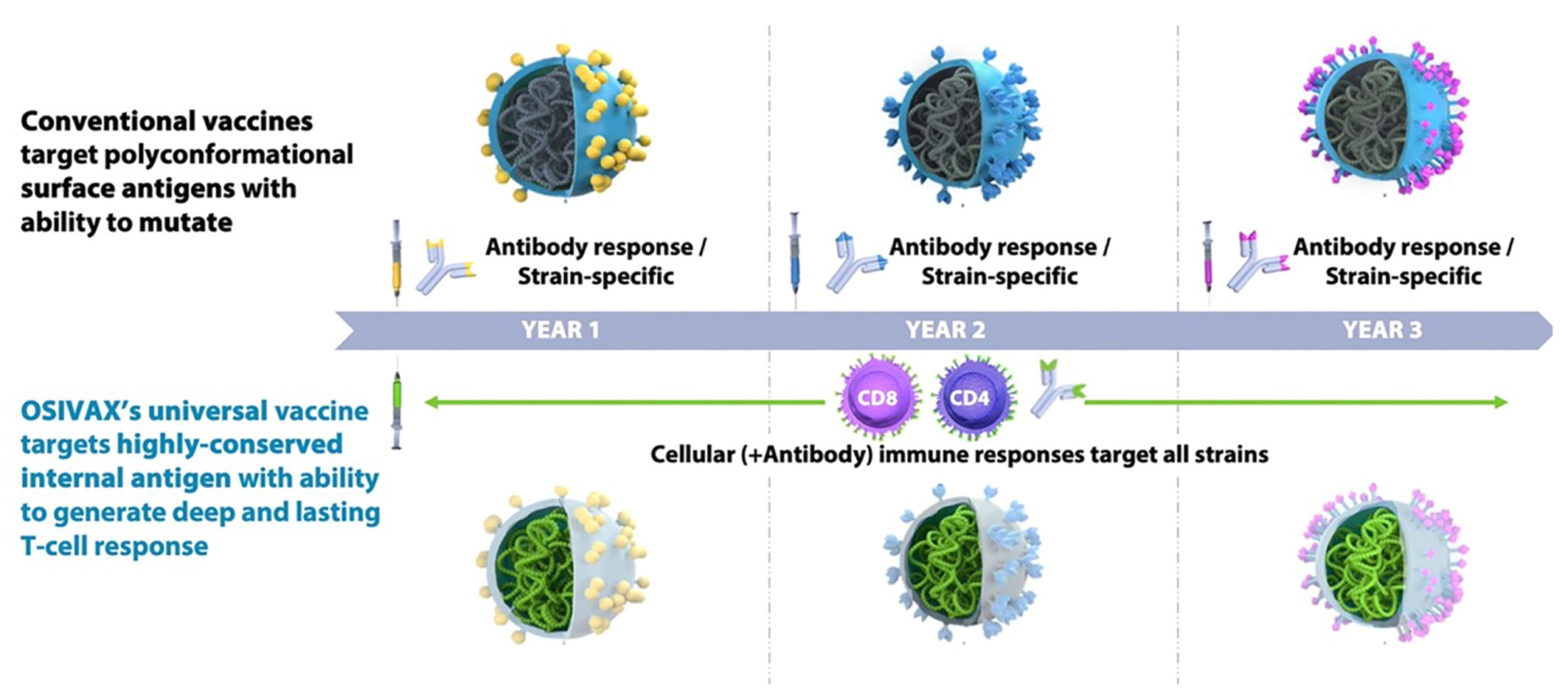

Figure 2: Osivax’ innovative oligoDOM® technology, an improved self-assembling nanoparticle approach, targeting internal and invariant parts of viruses2
In pre-clinical models, this approach demonstrated cross-protection against all A and B strains tested. In Osivax’ Phase IIa clinical trial in 300 healthy volunteers, OVX836 demonstrated an excellent safety profile, a robust and dose-dependent activation of the cellular component of the immune system, and a strong signal of protection against influenza symptoms.4 An additional Phase II clinical trial is planned to evaluate the co-administration of OVX836 with a standard QIV, empowering a double-punch approach to tackle the virus. Simultaneously, Osivax is advancing another broad-spectrum pre-clinical vaccine candidate against coronaviruses, which targets the nucleocapsid – an internal antigen of the SARS-COV-2 virus.
Conserved antigens, such as the nucleoprotein in influenza and the nucleocapsid in SARS-COV-2, have been recognised as significant targets for T cell-based broad-spectrum vaccine development. Combining a T-cell and B-cell response to tackle highly mutating viruses appears to be a promising means of providing enhanced efficacy in protection and cross-reactivity against all strains of a virus.
Upcoming developments
Broad-spectrum vaccines could provide robust protection against potential future outbreaks and/or pandemics of highly mutating viruses. While the emergence of COVID-19 certainly demonstrated the need for improved approaches to immunisation, it also showcased that progress in this area can be made rapidly when innovators work together.5 The vaccinology space remains one to watch as companies continue developing next-generation technologies, such as those focused on T-cell responses, intending to provide protection against viruses currently in circulation and those that are yet to come.
About the author
Alexandre Le Vert is the Chief Executive Officer and Co-Founder of Osivax. He has more than 20 years of experience in healthcare, ranging from research and development to commercialisation of vaccines and therapeutics. He is an experienced entrepreneur in biotech and innovation and currently serves as a Board Member of several biotech companies in France and the US. Alexandre is a graduate of EcolePolytechnique and Harvard University.
References
- Are more powerful vaccines coming? Shots targeting T cells show promise. [Internet]. Science. 2022 [cited 26 April 2022]. Available from: https://www.nationalgeographic.com/science/article/are-more-powerful-vaccines-coming-shots-targeting-t-cells-show-promise
- Technology – Osivax [Internet]. Osivax. 2022 [cited 26 April 2022]. Available from: https://osivax.com/technology/
- Madsen A, Cox R. Prospects and Challenges in the Development of Universal Influenza Vaccines. Vaccines. 2020;8(3):361.
- Leroux-Roels I, Waerlop G, Tourneur J, et al. Randomized, double-blind, reference-controlled, phase 2a study evaluating the immunogenicity and safety of OVX836, a nucleoprotein-based influenza vaccine [Internet]. Frontiers. Frontiers; 1AD [cited 2022Apr26]. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2022.852904/full
- We’re Getting Close to ‘Universal’ Vaccines. It Hasn’t Been Easy. [Internet]. Nytimes.com. 2022 [cited 26 April 2022]. Available from: https://www.nytimes.com/2021/12/08/magazine/universal-vaccines.html
Related topics
Antibodies, Biologics, Drug Development, Immunisation, mRNA, Research & Development (R&D), t-cells, Vaccine Technology, Vaccines, Viruses
Related organisations
Related diseases & conditions
Coronavirus, Covid-19, Influenza, Severe Acute Respiratory Syndrome (SARS)









A very well written article on a highly technical subject which even a layman like me can understand. The technical case is clear. But what about matters relating to economics? Is a broad spectrum vaccine affordable without Government subsidy? Would it just be another product for the rich?