A pragmatic approach for the adoption of QbD principles for analytical method development and validation
Posted: 18 April 2013 | | No comments yet
The principles of the application of Quality by Design (QbD) in the pharmaceutical industry in terms of development, manufacturing and control are well defined and described in the ICH guidelines Q8, Q9 and Q10. These guidelines mainly focus on the quality of the drug products, their manufacturing processes and the definition of a respective design space. The overall objective of QbD is to increase product understanding and to ensure a science and risk based approach in pharmaceutical development.


For each product, the Quality Target Product Profile (QTPP) summarises the respective quality characteristics. Critical Process Parameters (CPP) need to be defined, limited and controlled to ensure product quality.
Similar to product and process development, the efficient and effective ‘Quality-by- Design (QbD)’ approach can be applied to analytical method development. The goal of any analytical method development is to produce a robust and well understood analytical procedure which ensures reliability of the method during routine operation. The first adoption of QbD strategies to the development and validation of analytical methods is given in a position paper where the outcome of a joint effort of the PhRMA (Pharmaceutical Research and Manufacturers of America) and EFPIA (European Federation of Pharmaceutical Industries and Associations) working groups are conveyed1. In the context of analytical methods, the QbD approach consists of a collection of several aspects as shown in Figure 1.
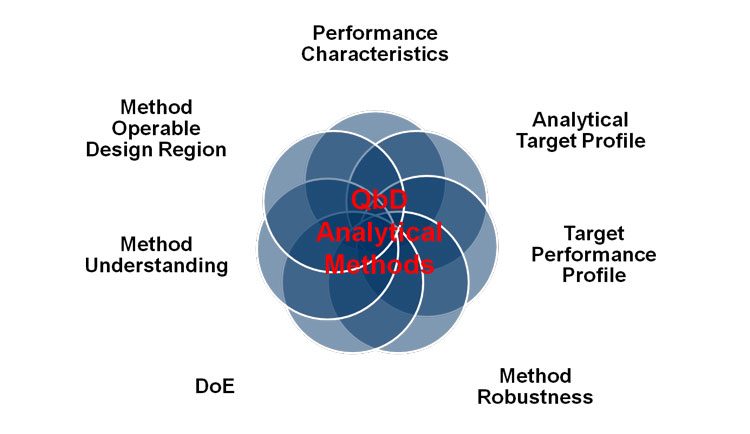

Figure 1: Aspects of QbD application for analytical method development
The current study reports the application and limitation of quality by design principles to chromatographic method development and validation. The objective of this study is to define the Method Operable Design Region (MODR) of an HPLC method for the determination of Benzalkonium chloride (BAC), which is used as a well-known preservative in inhalation solutions.
Questions such as the range to be covered and the required capability of the analytical method need to be answered during the development and validation of the analytical method, but the results of investigations concerning the chromatographic robustness also have a significant impact on the MODR. As an important aspect of those robustness studies, the influence of chromatographic parameters on peak separation needs to be considered during the validation process. Up to now, these investigations have been performed according to a One-Factor-At-A-Time approach in which one parameter was modified while all remaining parameters were held constant. The resulting range is to a certain degree hypothetical, as it does not consider simultaneous changes of different parameters at the same time which often happens in reality.
A very valuable instrument for those evaluations is Design of Experiments (DoE). This efficient method provides maximum information while, in theory, minimum experiments need to be performed. On the basis of the HPLC procedure for BAC, the selection of the investigated parameters on a risk based approach is justified and advice for the practical performance is given.
Theoretical development: analytical target profile
Prior to the method development, the Analytical Target Profile (ATP) was defined. The ATP for analytical methods can be regarded as the counterpart to the QTPP for pharmaceutical products.
The characteristics and criteria which represent the measurement requirements for the intended use of the method are formally summarised. This ATP is the basis for the following method development and validation process.
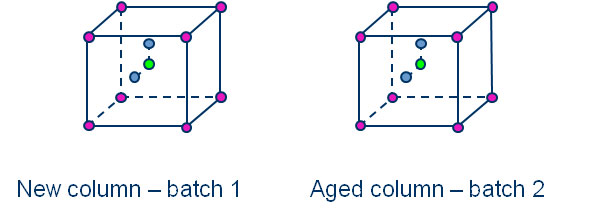

Figure 2: Graphical illustration of DoE setup
Target performance profile
- Assay of BAC in different formulations of inhalation solutions containing several active ingredients as well as further excipients.
Target performance characteristics
- Separation of the relevant alkyl substituents C12 and C14; Resolution (R) ≥ 1.5 Peak shape: Tailing (T) < 2.5
- Specificity to the additional components of the inhalation solution Accuracy: 98.0 – 102.0 per cent of recovery.
Chromatographic robustness
Typical parameters to consider during classic HPLC robustness testing in a One-Factor-At- A-Time (OFAT) approach are:
- Composition of the mobile phase
- Concentration of buffer solution
- Concentration of ion pairing reagent
- Column packings
- pH value of the mobile phase
- Flow rate
- Column oven temperature.
If all these parameters are taken into account during robustness testing applying (Statistical) Design of Experiments (DoE), this would result in numerous runs. Presuming six factors to be tested at two levels in a full factorial design, 26 = 64 experiments need to be done for a full evaluation.
It is very likely that during the on-going investigation, column aging will occur and the related change in chromatographic separation might cause an inconclusive statistical evaluation. Finally, separation changes cannot be precisely correlated to the robustness of the method or to the changed separation efficiency of the column. Therefore, it was necessary to limit the number of test parameters investigated to three to four on a risk based approach to ensure a reasonable size of the test scheme.
Thus, chromatographic parameters that are very good controlled for example by instrumental setting and qualification can be regarded as fixed and need not to be considered for the DoE. In the case of the BAC determination, the parameters column oven temperature and flow rate of the mobile phase were excluded from the DoE on this basis. Other parameters that are subject to accidental variations by handling, like weighing of the amount of buffer, were investigated. Also, the column can be a source of changes in chromatographic separation. In the present case, the following critical analytical method parameters were considered:
- Ratio of mobile phase composition
- Concentration of the buffer solution
- Concentration of ion pairing agent
- Different column batches with different ages.
Table 1: Ranges defined for set up of DoE
| Nominal value | Investigated range |
%-organic | 52 | 50 – 54 |
Buffer concentration | 11.60 | 11.50 – 11.70 |
Ion-pairing agent conc. | 2.20 | 2.10 – 2.30 |
Method robustness was examined by evaluation of the effect of small deliberate changes on these chromatographic parameters. The investigated range was chosen according to the variability that must be expected in practice during the preparation of the buffer solutions and the mobile phase. The variations from the nominal value were as follows:
Their influence on tailing and resolution, when varied in ranges of accidental variation, was monitored as indicators for quality of separation and peak shape according to the following test scheme presented in Table 2.
Table 2: DoE of the chromatographic robustness
Run | %-organic | Buffer concentration | Ion-pairing agent conc. |
1 | 50 | 11.50 | 2.10 |
2 | 50 | 11.70 | 2.10 |
3 | 50 | 11.50 | 2.30 |
4 | 50 | 11.70 | 2.30 |
5 (center point) | 52 | 11.60 | 2.20 |
6 (eccentric point) | 52 | 11.55 | 2.20 |
7 (eccentric point) | 52 | 11.60 | 2.25 |
8 | 54 | 11.50 | 2.10 |
9 | 54 | 11.70 | 2.10 |
10 | 54 | 11.50 | 2.30 |
11 | 54 | 11.70 | 2.30 |
The test design included the corners of the expected MODR as well as the centre point (run 5), representing the nominal chromatographic conditions.
Additionally, two eccentric test points (run 6 and 7) were included to ensure statistical modelling. The investigation was performed twice – separately for two batches of analytical column of different ages. For each run, two injections were made.
The set up can be illustrated by two cubes, where the dimensions in x, y and z direction represent the ratio of mobile phase composition, the concentration of the buffer solution and the concentration of ion pairing agent. Each cube itself stands for the test with one of the column batches with different ages.
With this design, a full quadratic regression model including main, interaction and quadratics effects could be used for each column batch.
Materials and methods: chromatographic conditions
The chromatographic parameters are summarised in Table 3. In Figure 3, a chromatogram of the BAC standard solution at standard conditions is shown. The first peak at retention time 5.3 minutes represents the alkyl substituent C12 while the alkyl substituent C14 shows up as second peak at retention time 12.9 minutes.
Table 3: Summary of chromatographic conditions
Chromatographic parameter | Setting |
Column | Inertsil® ODS-3, 100 x 3.0 mm, 3µm |
Mobile Phase | A: B 52 :48 (v/v) A: Acetonitrile |
Flow rate | 1.0 ml/min |
Column temperature | 30°C |
Detection | UV, 215 nm |
Injection volume | 50 µl |
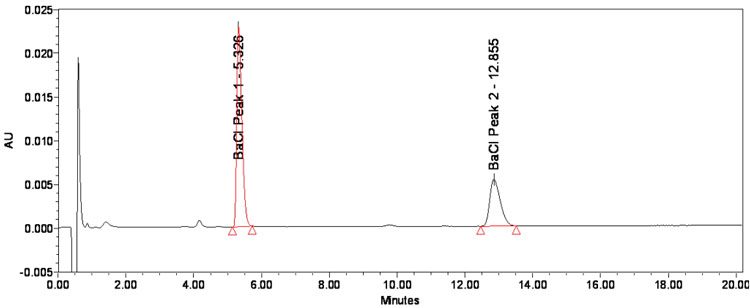

Figure 3: Chromatogram of BAC standard solution at standard conditions
Results of chromatographic robustness study
The DoE robustness study was performed according to the test scheme in Table 2. The influence on tailing and resolution was monitored as indicators for quality of separation and peak shape. All runs were clearly within the acceptance criteria for tailing and resolution as predefined in the target performance characteristics. That proves the chromatographic robustness within the borders defined.
Statistical evaluation of the DoE robustness study
The application of analytical robustness of DoE is quite different from ‘usual’ DoE approaches. For the latter, the objective is to reveal and detect relevant (and significant) models and effects, whereas for robustness DoE, it is desirable to have non-relevant (and nonsignificant) models and effects, with all observations and ideally also with all predictions and prediction limits within the desired specifications. In this case, the observed variability is only due to random, system-inherent variability leading, not to violations of the specifications.
In order to assess analytical robustness as well as to gain information on model and effects, the statistical analysis was performed in different steps:
- Main effect and interaction plots to detect relevant effects and obtain information on possibly critical directions
- Graphical display of predictions in contour plots to identify critical areas and directions
- Calculation of predictions and 95 percent prediction limits for the design area based on a full quadratic regression model for each column batch to assess analytical robustness.
The statistical evaluation shows that the ratio of the mobile phase composition has a large effect on peak separation, which decreases with increasing organic portion. Also, the column batch is an important factor on resolution, which can be clearly observed in the effect plot (Figure 4, page 40). The other parameters investigated showed only little influence on resolution as displayed in Figure 5 and 6.
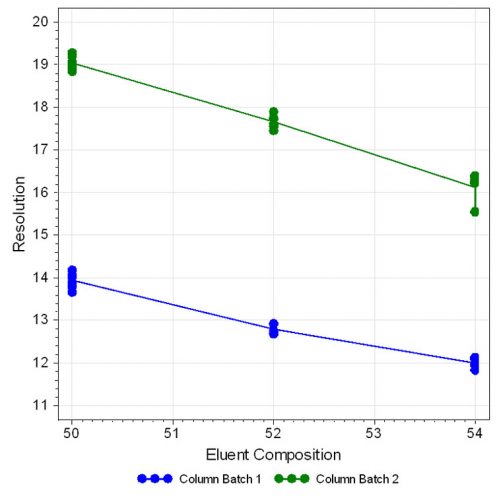

Figure 4: Effect plot for resolution with respect to eluent composition
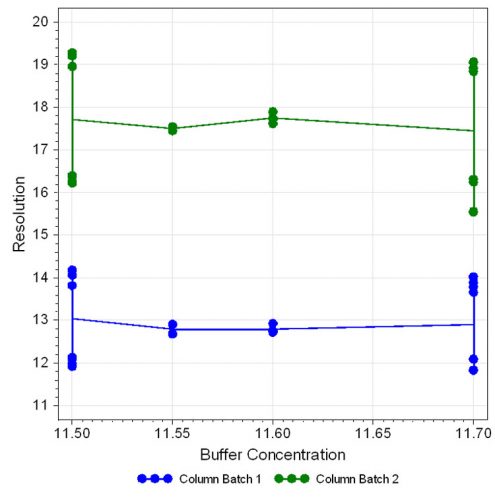

Figure 5: Effect plot for resolution with respect to buffer concentration
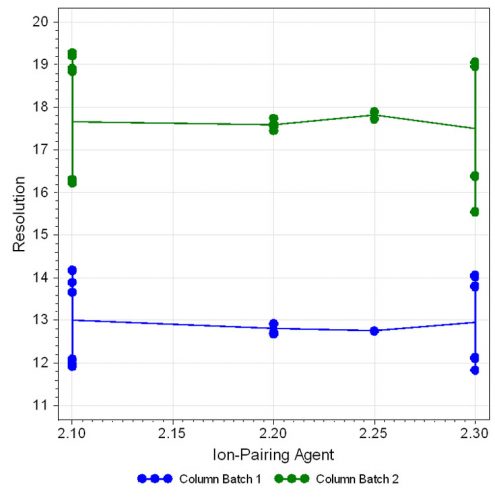

Figure 6: Effect plot for resolution with respect to ion-pairing agent
Additionally, interactions between the different critical chromatographic parameters can be assessed. The contour plots of the predicted resolution values show that the lowest resolution is to be expected when the highest concentration of buffer is combined with the nominal concentration of ion pairing agent at the highest portion of acetonitril in the mobile phase ratio (shown exemplarily for column batch 1 (new column) in Figure 7.
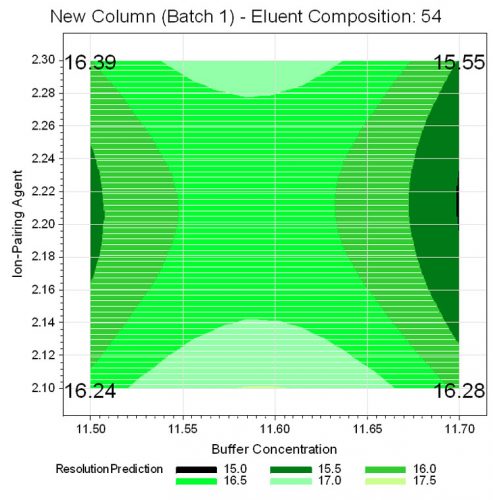

Figure 7: Contour plot for resolution for new column, batch 1
Table 4 (page 42) lists those factor combinations where the 95 percent prediction limits display the worst peak separation (R) and peak shape (T). The table shows again that the age of the column has the main influence on the results. The most critical factor combination regarding the acceptance criteria is found for the quality attribute T (Peak 2) for the aged column. The 95 per cent prediction limit is estimated as 2.38 for the combination of the chromatographic parameters, 54.00 per cent organic phase, 11.61 g/2l of buffer concentration and 2.21 g/2l ion-pairing agent. These settings were regarded as the ‘worst case conditions’, as additionally the lowest resolution values were observed for the aged column in general.
Table 4: 95% prediction limits for Resolution and Tailing
Column batch | Quality attribute | Prediction | 95% Prediction limits | Organic phase | Buffer concentration | Ion-pairing agent conc. |
New Column, Batch 1 | R | 15.2 | 13.72 | 54.00 | 11.70 | 2.20 |
T (Peak 1) | 1.7 | 1.69 | 50.00 | 11.60 | 2.10 | |
T (Peak 2) | 1.5 | 1.66 | 54.00 | 11.60 | 2.19 | |
Aged Column, Batch 2 | R | 12.1 | 10.35 | 52.40 | 11.70 | 2.30 |
T (Peak 1) | 2.0 | 2.10 | 52.20 | 11.70 | 2.30 | |
T (Peak 2) | 1.9 | 2.38 | 54.00 | 11.61 | 2.21 |
The study demonstrates that sufficient resolution is proven even when the extremes of critical chromatographic factors are combined. Applying these chromatographic conditions the accuracy was tested and the results for tailing and resolution were reconfirmed by real HPLC runs. Both column batches with different ages were tested and for each run, two injections were made. For the confirmation of the statistical prediction, again the acceptance criteria for tailing and resolution as well as for recovery were applied as predefined in the target performance characteristics.
The results gained for the peak shape were at about 1.8 and 1.7 for the two peaks determined with the aged column and comply very closely with the predicted values. This also can be observed for the new column. Also for the peak separation, almost the same value was gained during the experiment with the aged column (R=11.9) as previously predicted.
Real HPLC runs and predictions show a very good match to each other. Therefore, the choice and set up of the statistical prediction model is reconfirmed by the experiments. Nevertheless, the focus for the evaluation of robustness is set on the 95 percent prediction limits, as those values also include modelling uncertainty. The recovery found was in a range of 100.0 to 100.5 per cent, so that overall the acceptance criteria were met.
In the present example of the BAC determination, the statistical evaluation shows that the ratio of the mobile phase composition has a large effect on peak separation, which decreases with increasing organic portion. Also, the column batch and age is an important factor on resolution, which can be clearly observed in the effect plots. The other parameters investigated showed only little influence. Additionally, interactions between the different critical chromatographic parameters can be assessed. The lowest resolution is to be expected when the highest concentration of buffer is combined with the nominal concentration of ion pair reagent at the highest organic portion in the mobile phase ratio.
Discussion
The robustness of the method was evaluated with respect to variations of the mobile phase composition, the concentration of the buffer solution, the ion-pairing agent and of the column batch and age. The variations resulted in slight changes of resolution and tailing.
The most critical conditions were statistically calculated and reconfirmed in real HPLC runs for the determination of the recovery.
All requirements defined in the ATP were fulfilled and the chromatographic robustness and the robustness of the test procedure were proven. The MODR was therefore defined by the following ranges:
- Ratio mobile phase [%-organic]: 50 – 54 per cent
- Concentration of buffer: 11.50 – 11.70 g/2l
- Concentration of ion pairing agent: 2.10 – 2.30 g/2l
- Variation of column batch and age.
The application of QbD principles in method development and validation ensures the definition of a Method Operable Design Region. Within the MODR, the method is valid and reliable in any possible combination of the parameters varied.
Compared to the traditional approach for robustness testing, called ‘One-Factor-At- A-Time’, the DoE will deliver additional information about interactions of the chromatographic parameters. This includes simultaneous changes of the abovementioned parameters within the validated range, which are not investigated in the traditional approach. The most critical combination of the method parameters was identified by statistical evaluations and the predictions for quality attributes like tailing and resolution was calculated.
The confirmation of the prediction model by practical experience is essential. This ensures the appropriate setting of the MODR and therefore the reliability of the method in every day work.
Table 5: Verification runs of the statistical prediction
Column batch | Tailing factor BAC-Peak 1 | Tailing factor BAC-Peak 2 | Resolution | Recovery | ||||
Inj. 1 | Inj. 2 | Inj. 1 | Inj. 2 | Inj. 1 | Inj. 2 | Inj. 1 | Inj. 2 | |
New Column, Batch 1 | 1.5 | 1.5 | 1.4 | 1.5 | 14.2 | 14.3 | 100.0 | 100.1 |
Aged Column, Batch 2 | 1.8 | 1.8 | 1.7 | 1.7 | 11.9 | 11.9 | 100.4 | 100.5 |
Acknowledgements
The authors are thankful to Nicole Feuerbach for the experimental performance of the study and Holger Luley for the overall support and supervision.
Reference
- M.Schweitzer, M. Pohl, M. Hanna-Brown, P. Nethercote, P. Borman, G. Hansen, J. Larew Pharmaceutical Technology, Volume 34, Issue 2 (2010)
Biography








