A new challenge in the pharmaceutical supply chain
Posted: 27 March 2007 |
Current trends show that the Radiofrequency Identification (RFID) technology and Electronic Product Code (EPC) are experiencing increasing diffusion. Several business applications adopting RFID are currently expected to grow strongly. Bar codes and RFID will coexist for many years, although the former is likely to progressively replace the latter in some sectors faster than in others.
Current trends show that the Radiofrequency Identification (RFID) technology and Electronic Product Code (EPC) are experiencing increasing diffusion. Several business applications adopting RFID are currently expected to grow strongly. Bar codes and RFID will coexist for many years, although the former is likely to progressively replace the latter in some sectors faster than in others.
Current trends show that the Radiofrequency Identification (RFID) technology and Electronic Product Code (EPC) are experiencing increasing diffusion. Several business applications adopting RFID are currently expected to grow strongly. Bar codes and RFID will coexist for many years, although the former is likely to progressively replace the latter in some sectors faster than in others. The pharmaceutical sector is probably one of the industries most significantly impacted by RFID technology and will benefit greatly from its adoption. RFID can provide the track and trace functions essential to the public health chain and the prevention of drug counterfeiting too. Nevertheless, the extensive item-level tagging of products must go along with a reduction in technology costs, diffusion of standards and an improvement of end-user knowledge.
An overview of RFID technology and its impact on the pharmaceutical supply chain is presented in this article.
The adoption of Radio Frequency Identification (RFID) and Electronic Product Code (EPC) (Niemeyer and Pak, 2003) for product identification, as well as of EPCglobal Network (EPC Global, 2004) for information management, has recently been experiencing increasing diffusion in several business applications, such as transport and logistics, supply chain management, medicine and pharmaceuticals, manufacturing and processing, access control, real time location and agriculture, due to several reasons. From the technological point of view, RFID tags provide more accurate, punctual and updated information about products than traditional barcodes (Jones et al., 2004). For instance, manufacturing site, production lot, expiry date and component types are some of the information that can be linked to individual EPCs programmed in the tag chip. Such information can be usefully exploited to improve traceability of logistics processes, as well as monitor the performance related. Moreover, tags do not need line-of-sight scanning in order to be read, since they act as passive tracking devices, broadcasting a radio frequency when they pass within yards of a reader (Karkkainen, 2003).
RFID tags also solve some of the inefficiencies associated with traditional barcodes. For example, reading barcodes requires manual operations on packages, that is, either the packages with barcodes or the reading devices should be manually handled to read the codes (Boxall, 2000; Bylinsky, 2000; Jones, 1999). This may result in time consumption and difficult data capture if large amounts of goods are being handled. In some cases, readability of barcodes can be problematic too, due to dirt and bending, bringing about reduced accuracy and low reading rate (Ollivier, 1995; Moore, 1999). Based on these premises, RFID technology is expected to have a major impact on labour efficiency, automation and accuracy of many supply chain and logistics processes (Agarwal, 2001; McFarlane, 2002; Karkkainen and Holmstrom, 2002), in particular in such harsh environments as warehouses or distribution centers, where manual operations are critical. Although RFID technology significantly impacts on business process optimisation, the bottom line benefits for the supply chain are achieved through secure data sharing and punctual, real time and accurate visibility of EPC data through the logistics pipeline. The EPCglobal Network is the tool that enables paradigms for supply chain management (Cooper et al., 1997), as well as leanness and agility (Christopher and Towill, 2000) to become reality through faster and better-informed decisions. Focusing on the supply chain, real-time availability and sharing of EPC data through the EPCglobal Network are recognised as the main benefits of RFID and EPC implementations (Prater et al., 2005).
Real-time information availability implies additional outcomes, such as increased inventory visibility and stock-out reduction. Hardgrave (RFID Journal, October 14th, 2005) quotes that approximately 16 per cent stock out reduction have been achieved by Wal Mart through RFID deployment. Additional improvements enabled by EPC data sharing, quoted by scientific literature, are real-time access and update of current store inventory levels; automated Proof Of Delivery (POD) (Robertson, 2005); availability of accurate points of sale data; reduction of labour associated with performing inventory counts of shelved goods; improved theft prevention and shrinkage and better control of the whole supply chain (Bushnell, 2000). As far as the pharmaceutical supply chain is concerned, it must be noted that there are several additional aspects. RFID-enabled electronic product codes (EPCs) may be used to improve patient safety and process efficiency in the development of drugs and the running of clinical trials of drugs. Moreover, the new technology may also manage critical-care assets and hospital equipment and reduce counterfeiting and diversion of pharmaceutical products. Nowadays, counterfeiting is a growing and common problem affecting both governments and manufacturers around the world and is particularly dangerous when it involves medications (Figure 1).
Drug counterfeiting is not only an economic fraud, it also denies patients the therapies that can alleviate suffering and save lives. In February 2004, the Food and Drug Administrator (FDA) of the United States released a report entitled “Combating Counterfeit Drugs”. This comprehensive report highlighted the need for a combination of rapidly improving ‘track and trace’ technologies and product authentication technologies in order to provide a much greater level of security for drug products in the years ahead. In the same report, FDA highlighted that RFID tagging of products by manufacturers, wholesalers and retailers appears to be the most promising approach to reliable product tracking and tracing. Reliable RFID technology will make the copying of medications either extremely difficult or unprofitable. In the February 2004 report, FDA pointed out the importance of the adoption and common use of reliable ‘track and trace’ which would be feasible in 2007 and help secure the integrity of the drug supply chain by providing an accurate drug ‘pedigree’, i.e. a secure record documenting the drug was manufactured and distributed under safe and secure conditions. Given these premises, the present paper introduces an overview of RFID technology, its applications, the state of adoption and finally a case study: the RFID Lab project, recently set up at the University of Parma.
RFID technology basics
A typical RFID system (Figure 2) basically consists of four components: a host computer, readers, encoders and tags. Tags are made up of a microchip, no bigger than a grain of sand (approximately 0.3 mm2) and a flexible antenna embedded in a plastic-coated inlay having several forms and dimensions depending on the context and performance required. The information can be written onto the tags by an encoder printer, which are subsequently read by a reader that converts the electromagnetic wave pattern coming from the tag into digital signals and transmits them to the Information System by a terminal computer. Data are stored into the tag chip in the form of an Electronic Product Code (EPC). EPC standards have been developed by the Auto-ID Center, a partnership founded in 1999 by five leading research universities and almost 100 leading retailers, consumer product makers and software companies (Niemeyer et al., 2003). In the case of passive tags, probably the most interesting type for the supply chain RFID applications due to their low cost, the reader sends out an alternating electromagnetic wave pattern that is collected by the tag’s antenna, which converts it in both the energy and the information required for the tag to work. Owing to this double flow the tag can elaborate the information through the microchip and reply to the reader through its antenna with a new electromagnetic signal known as ‘backscatter reflection’. Passive tags are inexpensive, smaller in size, lighter in weight and have long lives. Their operative range is short and they have a limited memory when compared to the active tags. Active tags have an on-board battery and do not require an alternating electromagnetic wave pattern in order to send out a signal. They allow the reception and transmission of signals in long range tracking such as a football ground. Their cost is higher than passive tags and suitable applications are on ocean shipping containers, cars, valuable items and, in general, anywhere the high performance required justifies their high cost. Another type of tags is the semi-passive tag. Basically, these can be considered as passive tags that are small in size, lighter in weight and have limited memory, but in addition they have a battery backup that enables the answer range to be extended. Another important classification of tags is based on their data storage capability. More precisely, tags can be (i) Read/Written when data in the chip’s memory is updated; (ii) WORM (write-once read many) when data in the chip’s memory has been updated a limited number of times; (iii) Read only when update of data in the chip’s memory is not allowed and consequently identification processes only can be carried out. In order to help classify different tags, the Auto-ID Center defined an RFID Class Structure (Sanjay and Engels, 2003) to provide a framework for the standardisation of RFID tag functionality. The classes have changed over time, but the following is what was originally proposed:
- Class 1: tags are read-only passive identity tags
- Class 2: tags are passive tags with additional functionality such as memory or encryption
- Class 3: tags are semi-passive RFID tags. They may support broadband communication
- Class 4: tags are active tags. They may be capable of broadband peer-to-peer communication with other active tags in the same frequency band, and with readers
- Class 5: tags are essentially readers. They can power other Class 1, 2 and 3 tags, as well as communicate with other Class 4 tags and with each other wirelessly
Clearly the higher the performance of a tag, the higher the cost of the tag. A price comparable to a traditional label, which is lower than $0.035 (Bottani and Rizzi, 2007), will probably be the point beyond which the mass-market deployment of low-end tags will become viable. The current market cost for an active tag is a minimum of approximately $10 and approximately $0.25 – $0.40 for a passive one. Notwithstanding these data, the interest in RFID systems has increased significantly due to the trend of the costs. In other words, readers, tags and facilitating computers have rapidly dropped in price in the last few years and it is expected that the tag price will drop to $0.05 in a few years.
RFID applications
Just like human inventiveness, the applications where RFID technology can be implemented improving both the efficiency and the efficacy of processes is unlimited. Nevertheless, RFID technology is a tool and an enabler of functionalities: it is not a goal in itself (European Commission – Information Society and Media, 2006). Following the classification proposed by the European Commission, there are five main sectors that RFID applications can considerably impact on: (i) Supply chain management (ii) Transport (iii) Government (iv) Healthcare and pharmaceutical market (v) Modern mobile life
Supply chain management
Several studies have been conducted in order to assess both qualitatively and quantitatively the RFID technology impact on Supply Chain Management. Bottani and Rizzi (2007) have written a comprehensive and up-to-date report concerning a quantitative assessment of the impact of RFID technology and EPC system on the main processes of the Fast Moving Consumer Goods supply chain. In particular, results of the feasibility study show that RFID and EPC implementation is still not profitable for all echelons examined. These are manufacturers, distributors and retailers that are studied in both an ‘integrated’ and ‘non-integrated’ scenario. In the latter data are not shared between supply chain partners and the introduction of RFID in the pallet level tagging scenario provides positive Net Present Value (NPV) for all echelons examined. As far as the case level tagging scenario is concerned, it must be remarked that even if distributors and retailers achieve more benefit than the former scenario, manufacturers present negative NPV. Even though additional benefits are gained when an ‘integrated’ scenario is taken into account (i.e. data are shared between supply chain partners), manufacturers still present negative NPV when the case level tagging scenario is taken into account but positive NPV is obtained if the pallet level tagging scenario is considered.
Transport
Several benefits can be achieved by implementing RFID technology in transport and logistics. Object identification becomes much easier, more secure and efficient and, thanks to dynamic monitoring, a new management of the coming events can be implemented by forecasting arrival or departure times of trucks or any other resources. One of the promising prospects of deploying RFID in the transport sector involves quality monitoring through a real time centralised system of measures sensing the temperature or other features of perishable goods.
Government
Interesting applications in the public sectors can involve collection of statistical information in order to increase process transparency and control and support administrative processes. There is a large potential use of RFID in identification documents, banking and consumer-to-government interactions. For example, RFID can be included as an alternative technology to barcode for monitoring every pharmaceutical transaction along the supply chain. As reported in Wasserman, 2007 “the Italian government will soon unveil the first phase of a drug-tracking system designed to trace individual bottles of prescription medicine using bar code labels that have unique serial numbers from production to the consumer. It will require drug manufacturers, wholesalers and retail stores to report sales data to the Italian Ministry of Health within 24 hours of a transaction.” Although Italian pharmaceutical groups complain about the program, wholesalers such as Comifar Group believe the bar code is not the most appropriate technology and probably RFID could be more suitable.
Healthcare and pharmaceutical market
Several benefits can be achieved by implementing RFID technology in both the healthcare and pharmaceutical market. As far as healthcare is concerned, benefits can be gained by both patient and equipment tracking with RFID. In particular, RFID technology allows the automatic retrieval of relevant patients’ histories eliminating errors due to manual data entry. Moreover, real-time monitoring of both location and status of the patients can drastically improve their safety. By eliminating manual data entry requirements and enabling authorised staff to rightly access a patient’s status anywhere, both an increase in efficiency and a decrease in overall costs can be obtained. RFID-equipment tracking enables assets to be found instantly and their service ensured in a timely manner, thus increasing the patients’ safety. Moreover, efficiency goals can be achieved by eliminating the time wasted in finding equipment and increasing throughput of operating rooms. As far as the pharmaceutical market is concerned, several benefits associated with EPC/RFID adoption can be achieved concerning drug authentication, brand protection, drug supply chain efficiency and drug tracking along the supply chain. According to the World Health Organization, 5-8% of all drugs sold around the world are counterfeit, which represent between $7 billion and $26 billion of the $327 billion global market (International Chamber of Commerce, 2004). In order to secure the Healthcare & Life Sciences against counterfeits, an electronic pedigree based on RFID/EPC technology may make criminal drug diversion more difficult. This is a secure document that details data about each move a product makes along the supply chain, from the manufacturer through wholesalers to pharmacies and healthcare providers. Several mandates/guidelines trigger the establishment of an electronic pedigree system (EPCglobal US, 2005): (i) FDA strong endorsement of EPC/RFID as a product pedigree solution; (ii) strict state laws (Florida, California, Georgia, etc.); (iii) Prescription Drug Marketing Act (PDMA) Requirement; (iv) requirement from customers (Wal-Mart, Target, Albertson’s, Department of Defense, etc.); (v) Trade association agreements, such as Healthcare Distribution Management Association (HDMA). A further important benefit associated with EPC/RFID adoption is rapid and cost-effective recalls. In particular, both an increment in consumer safety and a reduction in costs associated with recalls executed with expensive manual processes can be carried out thanks to the possibility of instantly and automatically identifying the location of a product to be recalled (RFID White Paper Library, 2006). The loss in shareholder value after a drug recall is approximately 12 times the estimated cost brought about through litigation, recall, or replacement. In his comprehensive research Kearney (2004) estimates annual benefits of between $200 million and $400 million in avoided incidents of counterfeiting, with effects on both brand damage and public confidence. Thanks to RFID, less returned and wasted product can be achieved by a targeted recall saving $50,000 to $100,000 per year per $10 billion in revenue. Finally, Kearney estimates that overall economic benefits are between $500 million and $1 billion annually for pharmaceutical manufactures and between $200 million and $400 million annually for distributors.
Modern mobile life
From the combination of RFID and mobile handsets of the last generation, many existing services can bring benefits and a multitude of innovative applications can be deployed. Joining payments to mobile phones, automatically transferring a call to the office or meeting room where the phone’s owner is, monitoring visitors, monitoring the location of documents, retrieving product specifications from tagged goods or street posters (leading to online sales of products such as flowers, film tickets, DVDs, holiday trips) and guidance of poor-sighted citizens are some of the more evident examples in modern mobile life (European Commission – Information Society and Media, 2006).
State adoption
The increased interest and demand from the pharmaceutical industry is based on the promise of a safer and more accountable supply chain together with FDA support for technology (Ahlund, 2005). This feeling was substantiated by the European Commission, which in 2006 supported building the RFID solutions for the global environment (BRIDGE) project in the sixth framework program for research and technological development with €7.5 million. In recent years several pilots have been implemented by pharmaceutical manufacturers with different objectives and outcomes. In order to verify drug authenticity, Proude Pharma and the drug wholesaler H.D. Smith have implemented a pilot program based on RFID technology (G. Koroneos, 2005), whereas Cardinal Health in their pilot have demonstrated that it is feasible for UHF RFID tags to be inlaid into existing FDA-approved pharmaceutical label stock, and applied and encoded on case or pallet level for track and trace purposes at normal operational speed (McCormick, 2006). IMB have developed a complete product in order to supply a comprehensive system for tracking and tracing pharmaceutical products by combining RFID software, technology and services. Both Cardinal Health (Rios, 2006) and GSK (Van Arnum, 2006) have shown their interest in implementing pilot programs together with IBM. Finally, in order to combat the counterfeit product and enable pharmacies and wholesalers to verify the unique electronic product code, Pfizer have announced their intention to include an RFID tag on product packaging (Koroneos, 2006). In particular, in January 2007, Pfizer Inc began to ship Viagra® (sildenafil citrate) containing RFID tags to customers across the United States (Pfizer, 2007). In order to support these pilot programs and research activities, several RFID labs have been implemented in the last few years. Research activities reflect different interests in both theoretical and applied areas. Major typical topics concern Business Process Reengineering (BPR), technological tests and the evaluation of RFID impact on logistics and supply chain processes. Generally, in order to support these activities, RFID labs are equipped with both common logistic equipment, in order to reproduce real context in scale 1:1, and RFID equipment to test the actual response and performance of the new technology. RFID Labs usually join and partner academics, institutional and media partners, RFID technology providers, end users and stakeholders to foster research and technology transfer. In the next paragraph, the RFID Lab of Parma University, its structure, equipment, partners, current projects and activities are presented.
Parma RFID Lab
RFID Lab spun off by research carried out at the Department of Industrial Engineering of the University of Parma, in the field of RFID-based systems to manage the supply chain in the Fast Moving Consumer Goods (FMCG) and, more specifically, in the food industry. Starting in 2002, research projects have been studying the potentials of RFID for traceability, a mandatory EU requirement in the food industry (European Community, 2002; Rizzi, 2006), as well as its impact on supply chain processes in the FMCG. The center, founded and headed by Professor Antonio Rizzi, opened in May 2006. It is currently located in a 1,500 sq ft area at the Industrial Engineering Department of the University of Parma. The equipment currently installed at the Lab is mainly composed of a closed loop roller conveyor, a sorting system, a pallet conveyor, a wrapping machine, a storage system, a receiving dock, material handling equipment and IT equipment. The lab is the first Italian facility to be cleared by the Italian Ministry of Defense with a temporary site license to operate RFID equipment in the UHF 865-868 MHz spectrum (RFID Journal, 2006), according to ETSI 302 208 regulations (ETSI, 2006). RFID Lab is co-funded by Id-Solutions (www.id-solutions.it), a spin-off company of the University of Parma. Id-Solutions mission is to supply companies with RFID integrated solutions to automate and optimise logistics and supply chain processes. RFID Lab is thus not only a structure devised for academic research and education in the RFID field, but it also strives to become a national benchmark for technology transfer. With regard to technology providers, leading competitors in hardware and software solutions for RFID deployment have joined and coexist at the RFID Lab. The technology providers are: Id-Solutions, Avery Dennison, Caen RFID, EMS, Eximia, Fastweb, Hi-Pro, Intermec, Lab ID, Procomac Packaging, SAP, Siemens, Sirit, Sun Microsystems, Symbol, Tektronix, Telecom Italia, UPM, Microsoft and Oracle. Technology providers supply the RFID Lab with up-to-date equipment basically for a threefold reason: (i) testing HW and SW performance in a variety of supply chain conditions; (ii) showing the potentials of RFID hardware and software equipment to students attending programs at the RFID Lab, and (iii) having their products included in the research programs developed by the RFID Lab Board of Advisors (BoA). Institutional and Media partners support lab activities to different extents. Institutional partners encompass Emilia-Romagna Region, Parma Province and Parma City Council, Industrial association, AIM Italy. RFID Journal, Data Collection and RFID Italia are Lab Media Partners. A distinctive institution of the RFID lab is the Board of Advisors (BoA). The BoA is a steering committee set up to lead the research activities of the RFID Lab. Potential end users and stakeholders are involved in the BoA, which encompasses approximately 20 major Italian manufacturers, 3PLs and retailers: Apo Conerpo, Auchan, Barilla, Carapelli, Cavalieri Trasporti, Chiesi, Coop Italia, CPR System, Danone, Di-Tech, Grandi Salumifici Italiani, Glaxo Smith Kline, Lavazza, Number1, Parmacotto, Parmalat, Seda and Unilever. BoA members are Information, Logistics and Quality Executive Officers who do the following:
- Collaborate in drawing common research guidelines and programs
- Follow research programs and get research outputs and takeaways
- Have a reference point to learn about the latest trends and developments in the RFID field
As far as the research programs are concerned, two main research projects have been recently launched, namely ‘TT – Technology tests’ and the ‘RFID-DC’ projects. TT stems from BoA activities and basically aims at determining the performance that can be achieved with the adoption of up-to-date RFID technologies for products and packaging identification. Both HF and UHF fields are explored, although particular emphasis is given to UHF tests (EPC Class1 Gen2). The ‘RFID-DC’ research project aims to assess the technical feasibility of reengineered logistics processes of a representative FMCG Distribution Center through RFID technology. The models developed enable the quantitative assessment of the improvements brought in by technology in the FMCG industry. Finally, the RFID DC project strives to develop a proof of concept to substantiate the development of a pilot in the FMCG industry, involving manufacturers, 3PL and retailers’ production sites and distribution centers.
Conclusion
Nowadays there is some reserve to a wide and rapid diffusion of RFID technology because of its high cost and its potential to endanger consumer privacy and to reduce anonymity. As far as the pharmaceutical sector is concerned, there is still no evidence that the electromagnetic energy utilised by the reader device to read tags does not adversely affect the drugs exposed to it in terms of safety, potency, efficacy or changes in the pharmaceutical product’s composition. Notwithstanding the previous considerations, increased pressure for anti-counterfeiting initiatives comes from a growing number of states around the world and as stated by FDA, RFID product tagging by manufactures, wholesalers and retailers appears to be the most promising approach to reliable product tracking and tracing (Food and Drug Administration, 2004). Besides, over the last three years tag prices have fallen dramatically and this is only the starting point of a long-term trend. It is estimated that tag prices will decline on average 9 per cent per annum (Homs, 2004). RFID technology has the potential to revolutionise the world economy due to a new effective and efficient way to perform widely common operations. A wide range of applications can involve RFID technology and draw benefit from its adoption. Unfortunately, RFID technology currently remains a costly investment, but this is only a time issue.
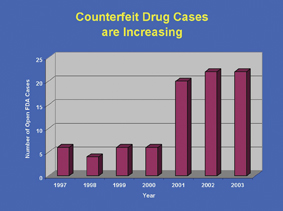

Figure 1: FDA open investigations 1997-2003 (SOURCE FDA)
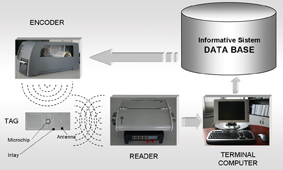

Figure 2: A typical RFID system
References
Karkkainen, M., 2003, “Increasing efficiency in the supply chain for short shelf life goods using RFID tagging”, International Journal of Retail & Distribution Management, vol.31, n.3, pp.529-536 Agarwal, V., 2001, “Assessing the benefits of Auto-ID technology in the consumer goods industry”, Cambridge University, Auto-ID Center. Available at http://www.autoidlabs.org.uk/ camwhitepapers.html Ahlund M. , Apr 1, 2005, RFID in the BioPharmaceutical Supply Chain, BioPharm International. BOTTANI, E., Rizzi, A., (in press), Economical assessment of the impact of RFID technology and EPC system on the Fast Moving Consumer Goods supply chain, International Journal of Production Economics, special issue RFID: technology, applications and impact on business operations Boxall, G., (2000), “The use of RFID for retail supply chain logistics”, paper presented at the Tag 2000, Baltic Conventions, The Commonwealth Conference & Events Centre, London, 24th May Bushnell, R., 2000, “RFID’s wide range of possibilities”, Modern Materials Handling, vol.55, n.1, p.37 Bylinsky, G., 2000, “Hot new technologies for American factories”, Fortune, vol.142, n.1, pp.288A-K Christopher, M., 1998, “Logistics and Supply Chain Management” Prentice Hall, London Collins, 2006, “Italian RFID Lab to Open in May”, RFID Journal, May 17th 2006. Available at http://www.rfidjournal.com/article/articleview/2204/1/1/ Cooper, M.C., Lambert, D.M., and Pagh, J.D., 1997, “Supply chain management: more than a new name for logistics”, The International Journal of Logistics Management, vol.8, n.1, pp.1-13 EPC Global, 2004, “The EPCglobal Network™: overview of design, benefits & security”. Available at http://www.epcglobalinc.org EPCglobal US, 2005, EPC Value Model for Healthcare & Life Sciences, ETSI, 2006, “Electromagnetic compatibility and Radio spectrum Matters (ERM); Radio Frequency Identification Equipment operating in the band 865 MHz to 868 MHz with power levels up to 2 W – Part 1: Technical requirements and methods of measurement”. Available at www.etsi.org European Commission – Information Society and Media, WORKSHOP ON: RFID application domains and emerging trends, POLICY FRAMEWORK PAPER, MAY 15-16, 2006, Available at: http://www.rfidconsultation.eu/ docs/ficheiros/Framework_paper_applications__ final_version_sw.pdf Food and Drug Administration, February 2004, Combating Counterfeit Drugs, U.S. Department of Health and Human Services. Homs, C., 2004, Exposing the Myth of the 5 cent tag, Available at www.forrester.com International Chamber of Commerce. Fact Sheet. Business Action to Stop Counterfeiting and Piracy. Paris, France. 2004 February. Available at http://www.uscib.org/docs/BASCAP_ factsheet.pdf Jones, L., 1999, “Working without wires”, Industrial Distribution, vol.88, n.8, pp.M6-M9 Jones, P., Clarke-Hill, C., Shears, P., Comfort, D., and Hillier, D., 2004, “Radio frequency identification in the UK: opportunities and challenges”, International Journal of Retail & Distribution Management, vol.32, n.3, pp.164-171 Karkkainen, M., and Holmstrom, J., 2002, “Wireless product identification: enabler for handling efficiency, customization and information sharing”, Supply Chain Management: An International Journal, vol.7, n.4, pp.242-252 Kearney A.T. and Healthcare Foundation. Adopting EPC in Healthcare: Costs and Benefits. Healthcare ,Distribution Management Association (HDMA). Reston VA 2004 November. Koroneos G. , Jan 12, 2006, Pfizer Combats Counterfeiters with RFID, ePT–the Electronic Newsletter of Pharmaceutical Technology. Koroneos G. , Jun 2, 2005, Purdue’s RFID Pedigree Program Enters Pilot Phase, Pharmaceutical Technology. McCormick D. , Nov 16, 2006, Cardinal Health Reports on RFID Pilot, ePT–the Electronic Newsletter of Pharmaceutical Technology. McFarlane, D., 2002, “Auto-ID based control”, White Paper, Cambridge University, Auto-ID Center. Available at http://www.autoidlabs.org.uk/camwhitepapers.html Moore, B., 1999, “Barcode or RFID: which will win the high speed sortation race?”, Automatic ID News, vol.15, n.7, pp.29-36 Niemeyer, A., and Pak, M.H., (2003), “Smart tag for your supply chain”, The McKinsey Quarterly, n.4 Niemeyer, A., M.H. Pak and S.E. Ramaswamy, 2003, Smart tag for your supply chain, The McKinsey Quarterly 4, 6-8 Ollivier, M., 1995, “RFID enhances materials handling”, Sensor Review, vol.15, n.1, pp.36-39 Pfizer, 2007, Pfizer Introduces Radio Frequency Identification Technology to Combat Counterfeiting, Protect Patient Health. Available at http://www.pfizer.com/pfizer/are/ investors_releases/2006pr/mn_2006_0106.jsp Prater, E., Frazier, G.V., and Reyes, P.M., 2005, “Future impacts of RFID on e-supply chains in grocery retailing”, Supply Chain Management: an International Journal, vol.10, n.2, pp.134-142 RFID Journal, October 14th, 2005, “EPC reduces out-of-stocks at Wal Mart”. Available at http://www.rfidjournal.com/article/articleview/1927 RFID White Paper Library, 2006 RFID and UHF: A Prescription for RFID Success in the Pharmaceutical Industry, WP-RFIDUHF Printed in USA 06/06, Available at http://www.rfidjournal.com/whitepapers/ download/127 Rios M. , Ago 24, 2006, IBM Develops RFID System for Pharmaceutical Track and Trace, ePT–the Electronic Newsletter of Pharmaceutical Technology. Robertson, I., 2005, “HP Samples Business Cases, EPCglobal Conference 2005, Atlanta GA, USA Sanjay E. Sarma and Daniel W. Engels. On the Future of RFID Tags and Protocols. Technical Report MIT-AUTO-ID-TR-018, Auto-ID Center, June 2003 Van Arnum V. , Mar 24, 2006, GSK Begins RFID Pilot Program, ePT–the Electronic Newsletter of Pharmaceutical Technology. Wasserman E. 2007, A Prescription for Pharmaceuticals, RFID Journal, Available at: http://www.rfidjournal.com/magazine/article/1739








