Is the dream becoming reality?
Posted: 28 November 2006 | | No comments yet
During the last decade, technical developments have dramatically changed the way cell-based assays could be implemented and used in research and development organisations. Although cell-based assays have moved into a modern era, cells are still grown and maintained in the same way as decades ago; i.e. manually. However, automation systems with the ability to grow and maintain cells have emerged, bringing us closer to the dream of fully automated cell culture.
During the last decade, technical developments have dramatically changed the way cell-based assays could be implemented and used in research and development organisations. Although cell-based assays have moved into a modern era, cells are still grown and maintained in the same way as decades ago; i.e. manually. However, automation systems with the ability to grow and maintain cells have emerged, bringing us closer to the dream of fully automated cell culture.
During the last decade, technical developments have dramatically changed the way cell-based assays could be implemented and used in research and development organisations. Although cell-based assays have moved into a modern era, cells are still grown and maintained in the same way as decades ago; i.e. manually. However, automation systems with the ability to grow and maintain cells have emerged, bringing us closer to the dream of fully automated cell culture.
Have you ever dreamed of an automated system that is able to grow the different cell lines maintained in your laboratory? If you have been involved with cell-based assay recently, particularly for high-throughput screening, the answer to this question is most probably ‘yes’. Over the last decade, a variety of scientific advances have brought cell-based assays to key strategic positions during the life of a therapeutic molecule from discovery to development. These include the growing number of potential targets revealed by the completion of the human genome project and recent advances in the field of proteomics, as well as the development of large compound libraries. The ever-increasing pressure to reduce development time and cost while enhancing commercial competitiveness in the pharmaceutical and biotech industries has also contributed to expending the use of cell-based assays1.
Cells are the closest representatives of physiological conditions that can be interrogated both in vitro in petri dishes and in vivo in xenograft models. Recent progress in the development of new cell lines, defined growth media and colorimetric, fluorescent or luminescent read-outs of multiple biological responses combined with significant improvements of readers, imaging and automation systems able to handle high-throughput assays now allow the implementation of specific and sensitive biologically-relevant cell-based assays. Such assays are being implemented at every step of a therapeutic molecule’s development from target identification and validation, primary screening, hit validation and lead profiling, mechanism of action and toxicity studies to therapeutic molecule production1,2. As a result, the need for a large number of cells is becoming the norm in the industry as well as in many academic organisations. Often the demand for cells exceeds the manual capabilities of even fully-dedicated technicians.
What is the answer?
Could automation of cell culture solve this bottleneck, as it has for high-throughput assays? The challenge here has been (and continues to be in some cases) the fact that cell culture work is often considered as a ‘black box’. Culturing cells is not a single-step, well-defined process but rather a succession of empirical tasks including cell dissociation, harvesting, seeding and feeding. Some cells are easier to grow than others, while some people are much better at growing cells than others. Could a robot therefore learn all these steps and grow healthy cells?
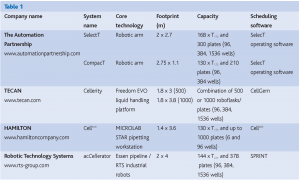
In 1998, taking advantage of progress in engineering of reliable and flexible miniaturised automation hardware and the development of elaborated software with the ability to simultaneously handle multi-component systems, six major pharmaceutical companies created aconsortium in collaboration with The Automation Partnership. The aim of the consortium was to develop and optimise the first fully automated cell processing system, named SelecT™. The first delivery of this system in 2002 was the beginning of a new era for cell culture. A fully automated system capable of growing, harvesting, counting, passaging and plating cells existed for the first time. Other automation companies soon followed with their systems: RTS with the acCellerator™, Tecan with the Cellerity™ and Hamilton with the Cellhost™. These systems are all built around different concepts, with different cell maintenance and plating capacities (Table 1). For example, the SelecT™ uses a robotic arm that mimics the motion of a human being to handle regular tissue culture flasks, whereas the Cellerity™ is built around a liquid handling system and requires specifically designed Roboflasks™. Each of these systems have the ability to grow and maintain cells in a fully automated fashion and have been purchased by various organisations for prices between 700 thousand and 1.5 million dollars. The outcome of their implementation depended largely upon how well prepared the end-users were to embrace this major change. Most users are cell biologists who recognise the opportunity to eliminate the cell culture bottlenecks in their cell-based strategies, while the decision-makers must first be convinced that cell culture automation is a viable option for their organisation.
A fallacy would be to consider these systems as turn key instruments that, upon installation, would be able to produce billions of your preferred cell lines. This is definitively not the case! Each system is custom-made and first requires the end-user to spend time reviewing the cells’ characteristics with the engineer in order to implement the best protocols on the system. Once the system is delivered, set up and sterilised, it usually requires significant adjustments in order to perform well for each cell line – a process that can take from three months to a year. Although the scientist may already have to share with the engineers how the cell should be handled, this information is based on the manual process and therefore pilot experiments must be performed on the robot. For each cell line the user has to optimise multiple parameters such as the cell dissociation buffer incubation time; the amount of shaking and knocking for cell detachment; the number of cells to be seeded per flask or the optimum number of flasks to be maintained in order to fulfill future demand. The optimisation of all these parameters requires time, mainly because the cells need time to grow. Contaminations could also occur within the system and significantly delay the implementation timeline since the system would have to be sterilised and the different cells re-entered again. In addition, technical glitches could also happen, especially early on during the implementation. These could compromise the continuous flow of cell culture and result in the loss of cells when, for example, cells have been harvested but not seeded by the system. Although these glitches are frustrating for the scientist, they provide exposure to learn how to recover from system errors. Finally, once the cells are routinely maintained on a given system, they must be validated by comparing their performance with cells maintained manually in the different cell based assays used downstream. This step is critical to convince the scientists that cells have been adequately maintained on the system across multiple passages.
Implementation
The implementation phase is usually the time when many scientists fear that they will never be able to run their system on their own. It is key to bear in mind when considering the purchase of these systems that they are complex pieces of equipment and, therefore, the involvement of an automation specialist as early as possible during the equipment selection, installation and optimisation processes is a major factor in ensuring successful implementation. While automation companies usually provide assistance during the set-up and the site acceptance test of their systems, having a dedicated in house automation specialist that is familiar with the system is critical to resolve glitches and re-optimise the system for additional cell lines, since some hardware adjustments or re-programming can be expected periodically. This reduces the dependence on the vendor service support and therefore decreases the chance of having the robot ‘down’ for too long. The lack of ‘in house’ technical support is usually the primary reason why companies fail to implement these expensive robots.
Personnel
How many full time employees need to be involved with an automated cell culture system? There is no doubt that these systems require expertise in two different fields: biology and automation. Since these robots are still in their infancy, it is rare to find an automation specialist with the proper cell biology background. Therefore at least two people with complementary expertise are required. One person must ensure that the system has all the consumables and media required and to introduce and monitor cell lines. The other is the automation specialist needed to program and trouble-shoot the system. The time spent by each of them on the system depends on the type of work performed on the platform. A system used to maintain one cell line for a large-scale high-throughput screening campaign will require less time from these individuals than one used to grow multiple cell lines for target validation.
The groups with adequate in-house automation support have benefited the most from their automated cell culture systems in multiple key areas. First of all, once a system is successfully implemented there is an immediate gain in productivity: the technicians who previously spent hours growing cells can instead spend their time working on less routine tasks. In addition, automated systems can handle more cell types than a dedicated technician, although, surprisingly, this benefit is not the most important for many groups. Rather, the improvement in cell quality is more important3. Human beings are not perfect and even for the most reliable technician cell maintenance could be challenging in term of consistency. There are always times when cell maintenance work would be postponed either at the end of a very long day or during a busy weekend. Unfortunately, cellular behavior and ultimately cell-based assay data could be greatly impacted if cell culture conditions change; for example, if the culture media is not replaced in time, or the cells are too confluent in their culture flask. Robots have the advantage of being extremely consistent, as well as not getting tired at the end of the day. Based on critical parameters such as number of cells seeded per flask, doubling time, maximum cell number per flask, an automated cell culture system will maintain the cells in a very controlled and consistent fashion over days and nights (including weekends), thus reducing stress on the cells and therefore reducing the inter assay variability.
Ultimately automated cell culture system implementation results in increased productivity and quality of cell-based outputs. However, these systems are not yet perfect. Except for cell number and viability, these systems are limited at providing qualitative data on the cells they maintain. Some systems such as the Tecan Cellerity can incorporate readers to automatically detect mycoplasma contamination, for example, but for any other parameters such as cell morphology, confluence or possible contamination the operator must still periodically inspect the flask manually. Future improvements will certainly include the integration of an automated microscope system able to independently monitor the cells, providing additional data that could be integrated by the software to determine the health status of the cells, as well as allowing the system to be interrogated remotely. Future software improvements should enable the current systems to be more proactively responsive to any changes in cell growth parameters. Several years from now these systems will likely evolve into more ‘intelligent’ robots with the ability to integrate multiple parameters and adequately adjust their operations.
Meanwhile, vendors will have to improve their instruments’ robustness, strengthen their service support, ensure extensive beta-testing of their new versions, while keeping user operations simple enough not to discourage potential new customers and keep their prices as competitive as possible. The dream of cell culture automation is therefore definitely becoming a reality. However, the field is still young and a few more years will be needed for the systems to mature and become a reality for many cell culture labs.

References
- S. A. Sundberg: High-throughput and ultra-high-throughput screening: solution- and cell-based approaches. Current Opinion in Biotechnology 2000, 11:47-53.
- E. C. Butcher, E. L. Berg, E. J. Kunkel: Systems biology in drug discovery. Nature Biotechnology 2004, 22: 1253-1259.
- J. Comley: Cell-based assay automation. Drug Discovery World 2005, 6:39-62.