Bottlenecks and potential improvements
Posted: 28 September 2006 | | No comments yet
Ion channels are membrane proteins that regulate the entrance and departure of specific ions from cells, thus influencing the physiology of all cells. These ion flows also underlie electrical impulses required for sensory and motor functions of the brain, control of contraction of heart, skeletal, smooth and vascular muscle, as well as nutrient uptake, hormone secretion, cell replication and foetal development.
Ion channels are membrane proteins that regulate the entrance and departure of specific ions from cells, thus influencing the physiology of all cells. These ion flows also underlie electrical impulses required for sensory and motor functions of the brain, control of contraction of heart, skeletal, smooth and vascular muscle, as well as nutrient uptake, hormone secretion, cell replication and foetal development.
Ion channels are membrane proteins that regulate the entrance and departure of specific ions from cells, thus influencing the physiology of all cells. These ion flows also underlie electrical impulses required for sensory and motor functions of the brain, control of contraction of heart, skeletal, smooth and vascular muscle, as well as nutrient uptake, hormone secretion, cell replication and foetal development.
Many pathophysiologies are associated with ion channel malfunctioning, for example cardiac dysfunction including life-threatening arrythmias, neurological disorders such as MS, epilepsy, neuropathic pain, diabetic neuropathy, and deafness. Therefore, controlled ion channel modulation would be of great therapeutic value in many diseases. Nevertheless, only some 5% of all known orally bioavailable drugs target ion channels, representing a current market value of around US$10 billion1. Given the relative under-exploitation of channels as a target class, there is vast potential in ion channel research for drug discovery and novel therapy development. Indeed, this relatively low percentage seems to have resulted from lack of appropriate starting points for medicinal chemistry and configuring relevant screening systems to identify novel chemical series for biased library formation.
So far, automated screening of ion channels is classically based on three main assay types: ligand binding, ionic flux or fluorescence. Ligands bind to the channel directly or to accessory proteins, thus modulating channel function. In ligand based assays (LBA) one identifies compounds which competitively displace ligand. However useful (e.g. sulphonylureas), this only allows for discovering compounds that act mechanistically in a similar manner as the ligand, thus restricting discovery of novel leads. LBA are relatively cheap however, allowing easily for 100,000 data points in 24 hours at relatively low cost per data point1. Ion flux assays measure the passage of radioactive ions through a channel of choice expressed in a cell line, but technical issues like requirements of cell washing to remove unloaded isotope, non-physiological cellular responses subsequent to stimulation of channel activation, and confinement to the 96-well microplate have hindered development of ionic flux assays for HTS. Fluorescent dye assays are also cell-based and typically measure changes in membrane potential or ion concentration, where throughputs of 50,000 compounds per 24 hours are now possible1.
Automated patch clamping
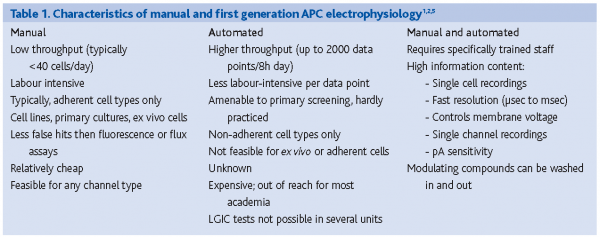
The above three assays types are all indirect measurements of ion channel activity. Also, the temporal response of most of these assays is greater than a second. Electrophysiology is the unquestioned standard for measuring the characteristics and activities of ion channels2. Since ions are charged particles, their flow through channels across a membrane causes an electric current to arise, which can be measured directly. Patch clamping is the classic manual technique to study ion channel behaviour, allowing for analysis under physiologically relevant timescales, voltages and ionic conditions with exceptional signal-to-noise ratios. Though the information content of electrophysiological data is higher than that derived from the biochemical assays, throughput is very low (Table 1).To be able to speed up the discovery process, machines that perform automated patch clamping (APC) are being developed. A few of these systems (‘first generation’) are now commercially available (for reviews see references 3-5). The throughput of these systems does not yet match the throughput of the biochemical assays described above1.
Currently, APC is indeed hardly used for primary screening of compound libraries in the pharmaceutical industry5. In the classical ion channel drug discovery process, electrophysiology only comes about during the phase of lead optimisation and preclinical stages1, likely because of its labour-intensive context. Given the nature of the data obtained with patch clamping, however, it would be highly desirable to integrate electrophysiological measurements at an earlier stage in the drug discovery process, to improve the data quality already at the primary screening stage. This can be achieved by continued miniaturisation and development of higher throughput machines that perform automated patch clamping.
Manual patch clamping can principally be performed on any electrically excitable cell, i.e. cells that are so by nature (such as neural or cardiac cells) or host cells transfected with – at least – the pore forming subunit of an ion channel. Additionally, small tissue slices can be studied. The APC systems so far have the disadvantage of only being able to measure suspension cells1,5, i.e. cells that grow in a non-adherent manner. The cells are washed into a so-called patch plate, where one cell per patch unit (well) is hoped to achieve a sufficient seal for measurement. The majority of channels under investigation are the voltage-gated types5 (VGIC) which are in nature not commonly expressed in such cells. This might increase the chance of false-positives or false-negatives in compound screens/tests since the biochemical environment of such cells is not naturally adapted to expressing VGICs. Results obtained with suspension cells as a model system in high throughput environments therefore require post-testing of the same channel subunit expressed in an adherent host cell type (e.g. HEK cells), or of cells obtained ex vivo from freshly isolated tissues known to express this channel natively (e.g. animal cardiac ventricular cells). Both follow-ups require manual patch clamping. Indeed, as long as screeners are willing to accept potentially losing some false-negatives and gaining some false-positives from transfected suspension cells4, the integration of direct APC measurements at an early stage of the drug discovery process linked to manual follow ups using other model systems for the ion channel under investigation may be a valuable approach to discover new leads that are chemically novel thus allowing for the construction of compound libraries that are biased around such new potential pharmacophore. In this manner, and with the aid of virtual screening6, theory-based and practice-based novel starting points for combinatorial chemistry arise, allowing for the composition of novel ion channel-specific libraries.
As already stated, the throughput of the first generation APC systems is insufficient to match primary screening criteria. In addition, of the currently available HT electrophysiology systems, costs per data point vary between US$0.75 and US$10 (see 5 for listing per vendor and APC product). Such costs may prove prohibitive for use in large scale screening. Indeed, Comley found that primary screening of full diversity libraries in ion channel drug discovery in the pharmaceutical industry only involves manual patch clamping for some 3% and APC for less then 5% of the respondents as the main technology. Primary screening of biased libraries only involves manual clamping for about 10%, and APC some 5% as main technology (2005-based questionnaire)5. The main technologies used for such ion channel target screens are fluorescence-based membrane potential assays (in both library scenarios some 40% of respondents) and fluorescence-based ion flux assays (in both screnarios some 30%). The main applications of manual patch clamping are found in the therapeutic area, i.e. target identification and validation studies (> 50%), and in safety assessment, e.g. hERG-compliant assays (50%). The main applications of APC are currently found in early non-compliant hERG liability testing (some 33%) and in secondary screening as well as hit-to-lead (lead optimisation) accounting in each case for some 25%. Respondents held the view that, in the next two years, APC is to impact mainly on early non-compliant hERG liability testing and compound selectivity profiling.
Moving from manual to automated
Some issues are of particular interest in the transformation from a manual to an automated protocol. Firstly, the quality of the seal of the patch clamp pipette with the cell membrane is of importance for success rates and for the signal-to-noise ratio of any measurement. In manual clamping practice, a resistance of at least 1-2 GΩ is accepted practice to start a measurement of ion channel activity. Here, a difference in viewpoint exists between staff with and without a strong background in electrophysiology, as unearthed by Comley: of those with a strong background in electrophysiology (‘patchers’) some 40% felt that minimum seal resistance required in a newly developed APC instrument should be 1-2 GigaOhm or higher, whereas more than 75% of those without such background (‘screeners’) would accept a minimum seal resistance of sub-GigaOhm values5 for screening purposes. Another issue, relevant for any cell-based assay, relates to the re-use of cells: should a cell after a successful electrophysiological measurement be replaced by another cell for follow-up measurements (different compound concentrations/different compounds), or is it acceptable to re-use cells as long as the seal is adequate? The predominant feeling in drug companies was that in safety assessment, only one compound should ever be tested at only one concentration for each cell preparation (some 60% of respondents), whereas for primary screening, compound profiling and early non-compliant hERG liability testing some 40% of respondents shared this feeling in each case. Primary screening seems viewed as the least exacting in its requirements with the majority of respondents stating – given proper seal resistance – they would continue testing the same cell by adding the same or other compounds. Interestingly, the trend showed that ‘patchers’ tend to opt more easily for re-using cells, whereas ‘screeners’ would be more strict in re-use of cells. Thus, diverse views exist among users of APC systems as to their mode of action in electrophysiological assays. Note that for currently available HT electrophysiology screening systems the costs per data point are lower when multiple tests are performed on the same cell preparation: from US$0.34 to some US$4 5 (cost reduction mainly because of re-using the expensive patch plate). Costs per data point could be further reduced by the development of appropriately high throughput patch plates. Currently, plates are 16 or 48 well, while 96 or 384 are desired5. Related to re-using cells is the ability of an APC system to bring on a carefully controlled laminar flow of fluid onto the cells to wash and/or bring on novel compounds. Measurements can be distorted by fluid turbulence, fluid front blurring and subsequent mechanical disturbances of the patched cell. Finally, the desired levels of accuracy in dose-response curves (IC50 determinations) obtained from APC measurements as compared to those obtained from manual clampings, are the least demanding for primary screening (up to a 10-fold difference in calculated IC50 would be acceptable) and the most demanding for safety assessment (up to a 2-fold difference would be acceptable). Current APC systems are inflexible in that they cannot be used for native or adherent cells (being again one more step further away from human physiology), and not all APC units offer fast and/or continuous laminar flow that is required for measurements of ligand-gated ion channels.
Good prospects for the future
So, it seems unlikely that high throughput electrophysiology will impact primary screening of ion channel targets in the short term, due to costs (consumables, instrumentation, maintenance) vs. budget restraints (out of reach for most academia), consequently costs per data point, and lack of desired instruments (e.g. cheaper patch plates of appropriately high throughput). Therewith the potential of the first-generation units to significantly shorten drug development times seems rather limited. Implementation of APC systems into safety assessment is also not likely on a short term, since this is a GLP-constrained context and by requirement the data generated by such machines must be widely accepted before change is considered, from validated assays4 (but see 7,8). APC systems will be expected to impact stages after primary screening, and the screening of smaller, biased high quality ion channel libraries. For novel routes in combinatorial chemistry directed at ion channel targets, it is of importance that APC systems would be integrated at an early stage of the drug discovery process, after primary screening of full diversity libraries (higher throughput). Doubts as to the quality of APC-generated data as compared to the manual methods can then be overcome by manual patch clamp follow up experiments, and other techniques, at a later stage (but obviously before committing significant combinatorial chemistry and resources to the ‘hit’) to verify APC data. With appropriate data quality, the use of HT electrophysiology will circumvent the disadvantage of manual clamping that the manual method usually takes place too late in the drug discovery process to have much impact on the design of novel and selective compounds1. And this was a major bottleneck in ion channel drug discovery: the inability to find molecularly different compounds to shape novel combinatorial chemistry pathways. But strategically, it is not only about numbers (albeit a key performance indicator), yet also about being able to predict which compounds have a good likelihood to survive the pipeline into the market9.
On the whole, prospects for HT electrophysiology are good: the APC market is predicted to grow with an estimated sales of more than 200 APC units globally in 2006, technological innovations relevant for APC are taking place10,11, and improved next-generation machines will surely impact turnover of HT electrophysiology machines and data quality5. Thus, novel areas of ion channel functionality research are likely within reach, hoping that the new and more rational than random approach will provide a palette of novel, selective and strong ion channel pharmacophores.

References
- Treherne JM. Exploiting high-throughput ion channel screening technologies in integrated drug discovery. Current Pharmaceutical Design 12 (4): 397-406, 2006.
- Jurkat-Rott K, Lehmann-Horn F. The patch clamp technique in ion channel research. Current Pharmaceutical Biotechnology 5 (4): 387-395, 2004.
- Wood C, Williams C, Waldron GJ. Patch clamping by numbers. Drug Discovery Today 9 (10): 434-441, 2004.
- Comley J. Automated patch clamping. Drug Discovery World Winter 2005/6: 62-79, 2005.
- Comley J. Patchers vs. screeners. Drug Discovery World Fall 2003: 47-57, 2003.
- Capelli AM, Feriani A et al. Generation of a focused set of GSK compounds biased towards ligand-gated ion-channel ligands. Journal of Chemical Information and Modeling 46 (2): 659-664, 2006.
- Guthrie H, Livingston FS et al. A place for high-throughput electrophysiology in cardiac safety: screening hERG cell lines and novel compounds with the Ion Works HTTM system. Journal of Biomolecular Screening 10 (8): 832-840, 2005.
- Sorota S, Zhang XS et al. Characterization of a hERG screen using the IonWorks HT: comparison to a hERG rubidium efflux screen. ASSAY and Drug Development Technologies 3 (1): 47-57, 2005.
- Federsel HJ. The integration of process R&D in drug discovery – challenges and opportunities. Combinatorial Chemistry and High Throughput Screening 9 (2): 79-86, 2006.
- Bugianesi RM, Augustine PR et al. A cell-sparing electric field stimulation technique for high-throughput screening of voltage-gated ion channels. ASSAY and Drug Development Technologies 4 (1): 21-35, 2006.
- Ionescu-Zanetti C, Shaw RM et al. Mammalian electrophysiology on a microfluidic platform. Proceedings of the National Academy of Sciences of the U.S.A. 102 (26): 9112-9117, 2005.
About the authors
Carlo Jochems
Carlo Jochems has several years experience with liquid handling biorobotics and High Content Screening using the Opera machine from Evotec for fluorescence cell-based assays. These were used to study ligand induced trafficking of GPCR-eGFP fusions in transfected cells, involving but not limited to the screening of a natural yet partly scaffold-designed compound library and a kinase inhibitor library. Pre-Opera, Carlo worked on a high(er) throughput liquid handling machine for the automated preparation of immunofluorescence microscope slides. Because of these experiences I am strongly interested in the use and logistics of HT technologies in life sciences.
Stefan Herzig
Stefan Herzig is C3 professor at the Pharmacology Institute of the University of Köln since 1995 and has long-standing experience with electrophysiological methods and the study of ion channels. He mastered his PhD in 1984 and habilitated in Pharmacology in 1992. In addition he obtained a Master in Medical Education in Bern in 2003 and also works as associate dean for medical education at the University of Köln. His research focuses on the behaviour, characterisation and pharmacology of T and L type calcium channels.




