The business benefits
Posted: 20 July 2006 | | No comments yet
So far in 2006 we have published contributions on a variety of PAT-related topics, including training (Issue 1), NIR (Issue 2) and the role of PAT in biotechnology (Issue 3). In this article Jean-Marie Geoffroy reports on the business case for PAT with his own interpretation and charts the road ahead.
So far in 2006 we have published contributions on a variety of PAT-related topics, including training (Issue 1), NIR (Issue 2) and the role of PAT in biotechnology (Issue 3). In this article Jean-Marie Geoffroy reports on the business case for PAT with his own interpretation and charts the road ahead.
So far in 2006 we have published contributions on a variety of PAT-related topics, including training (Issue 1), NIR (Issue 2) and the role of PAT in biotechnology (Issue 3). In this article Jean-Marie Geoffroy reports on the business case for PAT with his own interpretation and charts the road ahead.
PAT has been defined as:
systems for analysis and control of manufacturing processes based on timely measurements of critical quality parameters and performance attributes of raw and in-process materials
The PAT toolset consists of four basic elements. These elements are 1) modern process analysers, 2) process endpoint monitoring and control, 3) multivariate data acquisition and analysis, and 4) continuous improvement and knowledge management. Please note that items 1-3 are intended to improve our overall knowledge base, which can lead to (continuous) improvement in understanding and process control, item 4. (FDA, 2004)
Modern process analysers
The pharmaceutical industry has, at some level, always used modern process analysers – minimally from an R&D perspective and, in many instances, in a commercial facility. NIRs, Raman, TOC and many other instruments have been deployed in the field to monitor processing of APIs and drug products. This is especially true of drug substance manufacturers. In itself, this PAT toolset is not revolutionary but complementary to the real intent of PAT.
Endpoint monitoring and control
It is also true that process endpoint monitoring and control has also been carried out for a number of years for both drug substance and drug product manufacturers. However, the application of endpoint monitoring and control has usually been from a simplistic perspective. For example, granulation endpoint control through time, power or torque. Perhaps what is truly different from the previous paradigm for both modern process analysers and process endpoint monitoring and control is the degree of sophistication and deployment, as well as the use of multiple measurements for endpoint control. The use of a process trajectory to determine process control and endpoint is one relatively unexplored area for our industry. Methodologies are already available for the real-time control of multiple process parameters to ensure that they are adequately in control throughout the entire manufacturing process.
Multivariate analysis
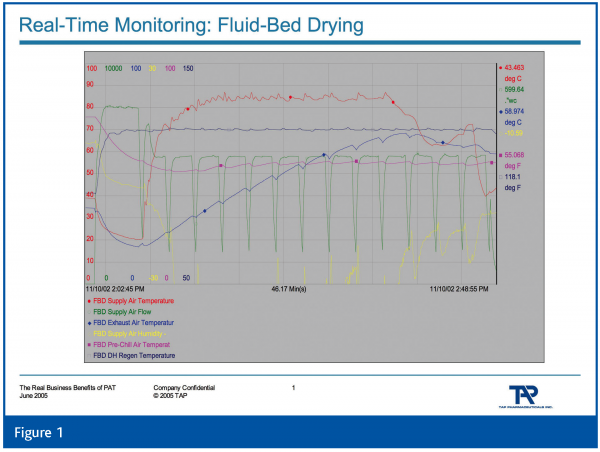
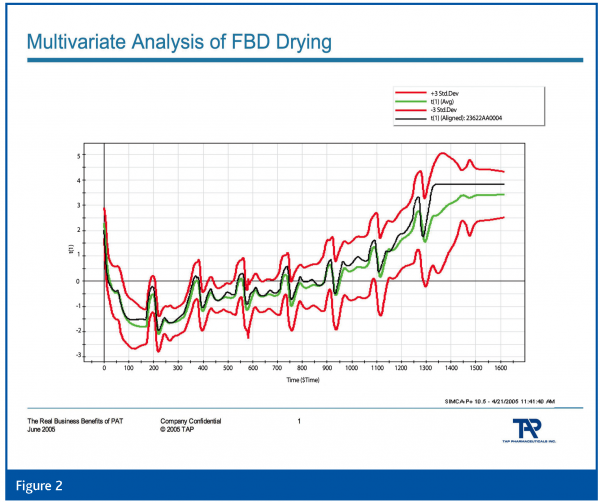
Of equal or greater interest are multivariate data acquisition and analysis, and continuous improvement and knowledge management. The former, in its most simplistic form, is the utilisation of multiple sources of data for understanding and control. Our industry has not evolved nearly as rapidly as other industries in the understanding and deployment of multivariate methods. See Figures 1 and 2 which demonstrate a typical fluid-bed drying process and the multivariate analysis of 8 process parameters using partial least squares analysis, respectively. Comparatively speaking, few in the industry understand the fundamentals of multivariate analysis and how to successfully deploy it; its pitfalls and opportunities. Perhaps academia can address this through new educational programs aimed at the pharmaceutical industry.
Continuous improvement and knowledge management
Continuous improvement and knowledge management involves the assimilation of all the available R&D and commercial data, analysing and learning on an ongoing basis. Modern analysers, endpoint monitoring and control, and multivariate analyses all serve to support continous improvement and knowledge management.
Since we can only improve that which we measure, careful selection of raw materials, in-process and release data is critical. In a previous study of a process which had difficulties with content uniformity, the author and colleagues engaged in the search for the root cause. Of the approximately 25 raw materials and process variables which were collected across 90 lots of product, and which consumed approximately 300 man-hours to collect from a paper batch record system over a weekend, none were useful in troubleshooting the problem. Clearly the batch data that was being collected was not useful in addressing this issue. No diagnostic measures were being collected as part of daily operations. Rather, new measures had to be put in place to determine the root cause.
Big picture: Quality by Design
What is Quality by Design?
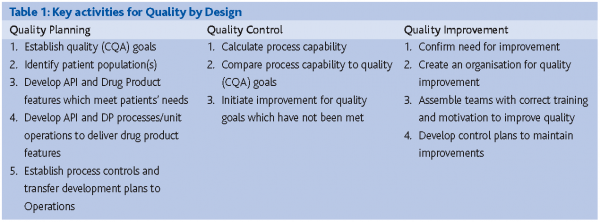
Quality by Design (QbD) is the process of managing for product quality.(J.M. Juran, 1992) Planning for Quality requires a different mindset for project execution. See The appropriate starting point for PAT is not always clear. Some organisations will consider Phase II or III as appropriate starting points, through commercial launch and support. QbD is much more holistic in nature, encompassing not only basic R&D CMC activities, but arguably market definition, patient identification, clinical indication and then all subsequent CMC activities. Therefore, QbD is much more comprehensive and value-added.
Planning for Quality means that the patient population has been clearly identified; their clinical needs have been adequately identified; the development of the dosage form is now geared towards that need and ideally dosage form properties can be predicted from basic and fundamental preformulation studies and knowledge about the dosage form delivery system itself (i.e. raw materials, unit operations, etc.) etc. This can be viewed as a return to an older paradigm where extensive preformulation was conducted as part of R&D when no models are avialable. From a business perspective, returning to longer timelines will not be acceptable. Therefore, it now also means predicting these properties a priori from the basic properties of the molecule based on theoretical, mechanistic and empirical modeling.
Recently, Helen Winkle of the FDA has asked that the ASTM E55 Committee consider widening its scope from PAT to broader activities, namely Quality by Design. (Helen Winkle, 2006) It is the author’s opinion that this recommendation is not only appropriate, but necessary. The QbD initiative has a much wider scope, consisting of cradle-to-grave R&D and commercial launch and support. The appropriate starting point for PAT is not always clear. Some organisations will consider Phase II or III as appropriate starting points, through commercial launch and support. QbD is much more holistic in nature, encompassing not only basic R&D CMC activities, but arguably market definition, patient identification, clinical indication and then all subsequent CMC activities. Therefore, QbD is much more comprehensive and value-added.
Drivers for change
The business drivers for an organisation to change are usually based on delivering products or services that increase the revenue of the company through growth or enhanced productivity. From a customer’s point of view, an enhancement in value can be achieved through improved quality, timeliness (products available sooner through rapid product development), additional functionality, enhanced services and relationships, or cost reductions. In the pharmaceutical industry, patient value translates to delivering higher quality products, sooner, with new features at a lower price. (R.S.Kaplan & D.P.Norton, 2001) The PAT toolset is designed to assist in meeting this organisational need.
From an operational level, however, the drivers for change become very much specific to the function providing the support. Perhaps the best advice I have received was to “talk money to the financial guys, quality and compliance to the quality guys and science to the science guys.” Acceptance of the justification for change is typically based upon one’s daily responsibilities. In many instances, a PAT project can deliver on financial, quality and science; however, the project champion may be selected based on the area of greatest return. For example, the vice president of operations is more apt to support a project with a significant return on investment rather than one based solely on quality, science or cost avoidance, whereas the vice president of quality is sensitised to compliance and a reduction in overall product variability.
At a very fundamental level, the main driver for any business change is financial. Securing funding for any project is significantly easier if tangible and direct business benefit can be demonstrated. A project based strictly on cost avoidance is typically only funded when the risk is high or very high; otherwise, such a project will get prioritised with all the other projects, and usually at a much lower position in the order than other projects based on real and immediate returns.
Advanced analytical technologies lead to a more fundamental understanding of physicochemical properties and process behaviour/trajectory. As a basic example, NIR technology has been used not only for characterisation of raw materials but also as a method for inline monitoring of chemical reactions, granulation processes, etc. These are significant advances over basic USP testing of raw materials, and time/power/torque based monitoring of granulation processes. Much more descriptive and quantitative information is available for decision-making. Many other technologies are being, and have been, developed.
The way forward
The way forward is to continue with PAT. The FDA has stated that there will be no other guidances relative to PAT, and that ICH Q8, Q9 and Q10 will become the documents to be cited for future guidance. PAT applications will become more commonplace, industry will become more comfortable with deployment of PAT applications, and those who have waited will follow suit.
From a scientist’s view, however, the new emphasis will be placed on the broader initiative of QbD. Much of what is QbD is often achieved intuitively or with an informal process. Minimally, QbD will mean redesign of CMC reports and applications. For some, it will also mean a complete redesign of how science is approached and conducted, requiring new skills and knowledge. Companies who best retain and communicate the knowledge and information they have generated will become the next generation of model companies.



References
FDA. Guidance for Industry
PAT – A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. 9-1-2006. Ref Type: Generic
Helen Winkle. ASTM E55 Opening Remarks to E55 Committee on PAT. 2006. Ref Type: Generic
J.M.Juran. Juran on Quality by Design: The New Steps for Planning Quality into Goods and Services. 1992. The Free Press. Ref Type: Generic
R.S.Kaplan & D.P.Norton. The Strategy-Focused Organization: How Balanced Scorecard Companies Thrive in the New Business Environment. 2001. Boston, MA, Harvard Business School Press. Ref Type: Generic








