Critical success
Posted: 24 March 2006 | | No comments yet
The extensive amount of knowledge accumulated over the last few years on the physiological importance of ion channels and, consequently, the broad expectation that drugs which modify the activity of these proteins could have therapeutic benefit, has triggered resurgence in the study of ion channels in both academic and pharmaceutical institutions.
The extensive amount of knowledge accumulated over the last few years on the physiological importance of ion channels and, consequently, the broad expectation that drugs which modify the activity of these proteins could have therapeutic benefit, has triggered resurgence in the study of ion channels in both academic and pharmaceutical institutions.
The extensive amount of knowledge accumulated over the last few years on the physiological importance of ion channels and, consequently, the broad expectation that drugs which modify the activity of these proteins could have therapeutic benefit, has triggered resurgence in the study of ion channels in both academic and pharmaceutical institutions.
Although the goal of researchers in academia is to understand the basic function of these proteins, while the pharmaceutical industry is more concerned with applied medical aspects, ideally both groups should be intimately connected. While the rationale design of ion channel drugs currently appears to be largely speculative, it should ultimately become a reality. Until that time, a plethora of different approaches are being used to identify molecules that could enter clinical development and eventually be accepted by Regulatory Agencies as drugs. Lead identification encompasses many different techniques and it is a necessary process that must take place before the more rational, structural-driven approach can drive the drug optimisation pathway. Therefore, development of high throughput assays with which to monitor functional ion channel activity represents an important, critical step in the process of drug discovery. However, even more relevant for successful efficacy readout(s) in clinical trials is proper target selection. The features of compound interaction with a particular ion channel/s in in vitro systems would become irrelevant if that channel is not involved in the physiological response/s that are trying to be modified in man. In the genomic era, the linkage between an ion channel and a disease is considered by many to be the rate determining step for later success in the clinic.
Ion channels are membrane proteins that can exist in different conformational states depending on the cell’s electrical environment in which they reside1, 2. They are critical regulators of essential physiological functions such as cardiac contractility, smooth muscle tone, nerve conduction, hormone and neurotransmitter release, water and electrolyte movement, cell growth and tumour progression. To accomplish these roles, cells express a wide variety of ion channels which function in coordination to regulate physiological signals. Changes in the activity of one of these proteins can alter the system’s equilibrium and cause a pathophysiological response. A classical example of this situation is the cardiac long-QT syndrome that can predispose an individual to the potentially lethal arrhythmia, torsade de pointes, which is caused by disfunction of either one of the two delayed rectifier potassium channels (hERG or KCNQ1) that participate in cardiac action potential repolarisation3. Long-QT syndrome can be the result of an inherited disease or due to an undesired drug interaction, either of which causes inhibition of one of these cardiac channels4. Although human genetics has helped to identify unambiguously whether a particular ion channel is selectively altered in a disease state3, it does not provide a convincing argument as to whether pharmacological intervention at that target would be relevant for treating that particular disease. If sulfonylureas had not been found to lower blood glucose serendipitously during antibiotic development5, their clinical application for the treatment of Type 2 diabetes would have been questionable – especially given what is known about the phenotype of individuals that have mutations in the ion channel protein (KATP), which is the target of these agents6, or the phenotype of the mice in which the KATP channel is knocked out7, 8. In most cases, however, the contribution of ion channels to cell function is not absolutely known and, even more concerning, the actual expression profile of channels in specific cell types is yet to be characterised. Peripheral sensory neurons express many types of voltage-gated sodium channels that are thought to be involved in initiation and transmission of pain stimuli after nerve injury9. However, the particular channel(s) that is involved in pain signaling after injury is still to be convincingly defined, with different experimental approaches apparently yielding conflicting results.
It appears that target validation will require several complementary approaches which may include, but are not restricted to, genomics, proteomics, transgenic animals, siRNA application, antibody probes, electrophysiological measurements, immunolocalisation studies and pharmacological assessment. Ideally, more than one approach should be used for proof of concept studies to ensure the chances of success later in the drug development process. Pharmacological tools are perhaps the most relevant to drug discovery because they can provide a more complete understanding of the function of a given target. For instance, while genetically altered animals, siRNA and antibody reagents can identify important components of a system’s biology, the actual channel target could also contain other ion channel regulatory subunits that may play important roles in conferring unique biophysical and/or pharmacological properties to the ion channel complex. In this respect, the high-conductance calcium-activated potassium (Maxi-K) channel has been proposed as a target for development of novel smooth muscle relaxant agents. Smooth muscle Maxi-K channels are formed by the association of the pore-forming β subunit and a β1 auxiliary subunit, which is not expressed in other tissues where the β subunit is present. In the presence of β1, Maxi-K channels become major contributors to smooth muscle tone and their pharmacological properties can be significantly altered10. Obviously, assays designed to identify Maxi-K channel agonists for smooth muscle relaxant applications will need to consider the relevant channel composition and use, as a positive control, of a pharmacological agent that reflects target validation. For ion channels in general, these pharmacological agents can be either small molecules or selective ion channel modifying peptides isolated from venom of poisonous organisms.
One of the advantages of using pharmacological tools in target validation resides in the possibility of being able to design in vivo experiments and determine the consequences of ion channel modulation at the level of the whole organism. Although the results of preclinical studies and clinical efficacy may not necessarily agree, in vivo proof of concept studies provide a margin of comfort before assigning a significant amount of resources to a project. There are substantial in vitro data to suggest a role for the voltage-gated potassium channel, Kv1.3, in calcium-dependent T cell activation processes. With the use of peptides that, under defined experimental designs, selectively block this channel, it was possible to determine that Kv1.3 represents a therapeutic target for treatment of autoimmune diseases such as multiple sclerosis11, and that it is also a target for preventing graft rejection after organ transplantation12. Similar results were later found with the use of small molecule inhibitors of the Kv1.3 channel13. In the course of these studies, the importance of using appropriate animal species that mimic human physiology was also realised. Because the complement of ion channels in rodent T cells is quite different from that of humans, rodents do not represent a relevant model for predicting the efficacy of Kv1.3 inhibitors in man. Thus, evidence for the presence of an ion channel of interest in the human target tissue should be seriously considered at the early stages of drug discovery. Even when the channel is present in tissues of interest, there can be significant functional differences (i.e. differences in electrical signaling) across species that mandate a more thorough evaluation of a channel’s contribution to cell physiology.
When enough evidence is accumulated to support validation of a given target, the actual process of drug development can be put in place. If no lead structures are available, then the initial goal is to identify screening actives with appropriate characteristics that can be further improved by medicinal and/or combinatorial chemistry efforts into a lead candidate. Further refinement to optimise for drug metabolism and pharmacokinetic properties will provide a compound that can be evaluated in preclinical safety assessment studies before entering clinical development. To support the identification of positive screening hits, as well as to be able to support chemistry efforts, high throughput assays must be established. Technology has significantly advanced in the last few years to make the functional high throughput screening of ion channels possible. Because these proteins selectively permeate ions, their activity can lead to cell membrane hyperpolarisation or depolarisation, depending on the ion channel under consideration, and actual assay design. Therefore, different techniques have become available to measure the function of ion channels in a reproducible manner. Each technique has its positive and negative aspects and, in the best case scenario, it is preferable to use more than one assay to adequately evaluate the effect of identified ion channel modulators.
The gold standard for measuring ion channel activity is electrophysiology, since it is a direct readout of function, provides high resolution and has the flexibility of allowing changes in assay parameters as the experiment progresses. The mechanism of action of a compound is best determined using this experimental technique and this information can be helpful for making predictions about safety issues and the therapeutic index that can be expected given the features of drug-channel interaction. However, manual electrophysiology is a time consuming process that only affords testing of a limited number of compounds daily. To increase the throughput, several commercial automated patch clamp systems have become available in recent years. Many technical features distinguish these different platforms which makes each one optimal for certain types of applications. Information about this technology has been reviewed extensively in recent issues of European Pharmaceutical Review14. However, despite significant advances in this technology, even the highest throughput automated electrophysiology instrumentation currently available will not be able to support campaigns that involve sample collections of over a million compounds. Although it is possible that instruments with that capability may become available in the future, at the present time other assays must be implemented as primary ion channel screens to assay large sample collections.
The inherent property of ion channels of being able to modify the membrane potential of the cell in which they reside has been successfully exploited for developing high throughput functional assays. The use of fluorescence dyes that partition within the membrane according to the membrane potential is gaining attention because they provide high capacity, relatively medium cost and good assay reproducibility. In particular, fluorescence resonance energy transfer (FRET) between two fluorophores, a donor that resides at the outer leaf of the membrane and an acceptor, a mobile lipophilic anion that distributes within the membrane according to the membrane potential, has been successfully employed to establish functional assays for a variety of voltage-gated channels, transporters and ion pumps15. When conditions are properly selected, good correlation for compound potency between FRET-based membrane potential assays and electrophysiological assays can be observed. This is the case for the voltage-gated sodium channels Nav1.5 and Nav1.7 stably expressed in HEK293 cells where channel-dependent depolarisation is triggered by addition of the sodium channel agonist veratridine16. Because the membrane potential of these cells is quite depolarised (i.e. ~ -50 to -40 mV), most channels will reside in an inactivated state that favours high affinity interaction with clinically relevant state-dependent blockers. Addition of the sodium channel modulator veratridine, which removes fast inactivation, allows the flow of sodium ions through open, unmodified channels causing the cells to depolarise to the sodium equilibrium potential with a consequent change in the FRET signal. In the presence of an inhibitor, the number of channels in the open and resting states that are available for veratridine modification is reduced and the change in FRET signal is attenuated. By controlling the amount of veratridine that is used to trigger sodium channel-dependent cell depolarisation, it is possible to achieve optimal sensitivity for detecting inhibitors in this type of assay. The possibility of using plate reader platforms compatible with a high density format, such as 384- and 1536-wells, makes the FRET-based assay for Nav1 channels a primary screen for lead identification and Medicinal Chemistry support.
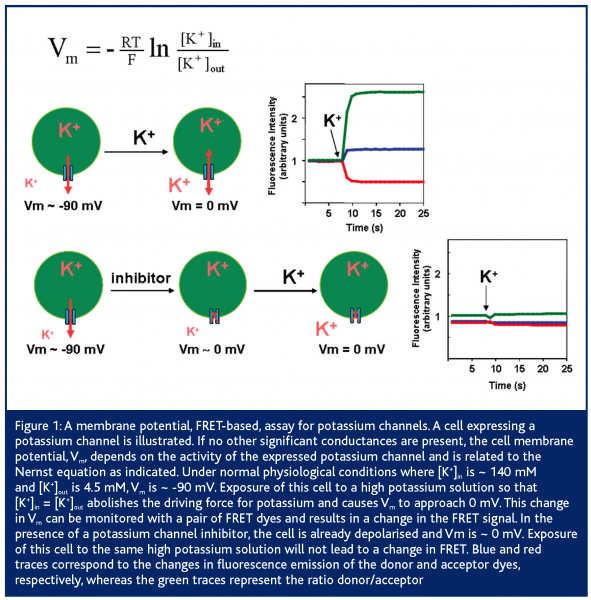
Membrane potential based assays can also be used to monitor the activity of potassium channels, and FRET-based assays provide optimum compound evaluation owing to the fast response of the dyes17. In this case, a given potassium channel is expressed in a cell line so that it becomes the dominant conductance and sets the resting potential of the cell (Figure 1). Depending on the biophysical properties of the channel, different levels of channel expression will be needed to accomplish this goal. Addition of a high-potassium solution will abolish the driving force for potassium leading to cell depolarisation and a change in FRET signal. In the presence of a potassium channel inhibitor, the cell will already be depolarised and no change in FRET signal will occur upon addition of the high-potassium solution. It is also possible to format this assay for finding potassium channel agonists that will cause an increase in FRET signal due to cell hyperpolarisation to EK (~ -90 mV). Unlike the situation with sodium channels, compounds in potassium channel assays could appear to be less potent than in conventional electrophysiology assays. The reason for this resides in the fact that membrane potential is a non-linear function of channel activity, and that a significant number of channels may have to be blocked to cause cell depolarisation. Because cells tend to over-express these channels, care should be used to select cell lines that have appropriate channel density to provide a robust signal without compromising compound potency.
Research activity directed at developing novel FRET systems that could be used to record physiological functions, such as the visualisation of action potentials, is ongoing. Recently, the use of genetically encoded fluorescence voltage probes based on farnesylated enhanced green fluorescence protein (eGFP-F) has been reported18. In this system, a synthetic voltage-sensing molecule, dipicrylamide, translocates across the membrane in response to changes in membrane potential and, although it is not fluorescent, it can absorb energy from eGFP-F, attached to the inner leaflet of the membrane through a farnesylation tag. This experimental system which provides a large fractional fluorescence change with a fast response time and large dynamic range has shown utility in measuring trains of action potential spikes in primary neurons. By expressing eGFP-F under the control of a cell specific promoter it should be feasible to image specific neuron populations. As an alternative approach, the use of fluorescence labeled antibodies against cell specific proteins in combination with dipicrylamide could provide a readily available system for probing many cell functions.
The process of drug development begins with lead identification. For ion channels, target validation appears to be critical for later success in clinical trials. Technologies to study the function of ion channels have emerged and are constantly being improved to provide high fidelity and high capacity screens. It is expected that, in the coming years, a large number of ion channel modulators will be brought into clinical development and, most importantly, some of them will succeed in becoming drugs that will improve the quality of life of patients.

References
- Long SB, Campbell EB & MacKinnon R (2005). Voltage Sensor of Kv1.2: Structural Basis of Electromechanical Coupling. Science. 309, 903-908.
- Long SB, Campbell EB & MacKinnon R (2005). Crystal Structure of a Mammalian Voltage-Dependent Shaker Family K+ Channel. Science. 309, 897-903.
- Ashcroft F, Ion Channels and Disease. 2000: Academic Press.
- Fermini B & Fossa AA (2003). The impact of drug-induced QT interval prolongation on drug discovery and development. Nat Rev Drug Discov. 2, 439-447.
- Li S, Gosling M, Poll CT, Westwick J & Cox B (2004). Therapeutic scope of modulation of non-voltage-gated cation channels. Drug Discov Today. 9, 1045-1054.
- de Lonlay P, Giurgea I, Sempoux C, Touati G, Jaubert F, Rahier J, Ribeiro M, Brunelle F, Nihoul-Fekete C, Robert JJ, Saudubray JM, Stanley C & Bellanne-Chantelot C (2005). Dominantly inherited hyperinsulinaemic hypoglycaemia. J Inherit Metab Dis. 28, 267-276.
- Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L & Bryan J (2000). Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem. 275, 9270-9277.
- Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J & Seino S (1998). Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 95, 10402-10406.
- Novakovic SD, Eglen RM & Hunter JC (2001). Regulation of Na+ channel distribution in the nervous system. Trends Neurosci. 24, 473-478.
- Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H & Pongs O (2000). Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res. 87, E53-60.
- Beeton C, Pennington MW, Wulff H, Singh S, Nugent D, Crossley G, Khaytin I, Calabresi PA, Chen CY, Gutman GA & Chandy KG (2005). Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol Pharmacol. 67, 1369-1381.
- Koo GC, Blake JT, Talento A, Nguyen M, Lin S, Sirotina A, Shah K, Mulvany K, Hora D, Jr., Cunningham P, Wunderler DL, McManus OB, Slaughter R, Bugianesi R, Felix J, Garcia M, Williamson J, Kaczorowski G, Sigal NH, Springer MS & Feeney W (1997). Blockade of the voltage-gated potassium channel Kv1.3 inhibits immune responses in vivo. J Immunol. 158, 5120-5128.
- Koo GC, Blake JT, Shah K, Staruch MJ, Dumont F, Wunderler D, Sanchez M, McManus OB, Sirotina-Meisher A, Fischer P, Boltz RC, Goetz MA, Baker R, Bao J, Kayser F, Rupprecht KM, Parsons WH, Tong XC, Ita IE, Pivnichny J, Vincent S, Cunningham P, Hora D, Jr., Feeney W, Kaczorowski G & et al. (1999). Correolide and derivatives are novel immunosuppressants blocking the lymphocyte Kv1.3 potassium channels. Cell Immunol. 197, 99-107.
- Herrington J, McManus O & Kiss L (2005). The road ahead and how to get there. European Pharmaceutical Review. 10, 79-84.
- Gonzalez JE, Oades K, Leychkis Y, Harootunian A & Negulescu PA (1999). Cell-based assays and instrumentation for screening ion-channel targets. Drug Discov Today. 4, 431-439.
- Felix JP, Williams BS, Priest BT, Brochu RM, Dick IE, Warren VA, Yan L, Slaughter RS, Kaczorowski GJ, Smith MM & Garcia ML (2004). Functional assay of voltage-gated sodium channels using membrane potential-sensitive dyes. Assay and Drug Development Technologies. 2, 260-268.
- Middleton RE, Sanchez M, Linde AR, Bugianesi RM, Dai G, Felix JP, Koprak SL, Staruch MJ, Bruguera M, Cox R, Ghosh A, Hwang J, Jones S, Kohler M, Slaughter RS, McManus OB, Kaczorowski GJ & Garcia ML (2003). Substitution of a single residue in Stichodactyla helianthus peptide, ShK-Dap22, reveals a novel pharmacological profile. Biochemistry. 42, 13698-13707.
- Chanda B, Blunck R, Faria LC, Schweizer FE, Mody I & Bezanilla F (2005). A hybrid approach to measuring electrical activity in genetically specified neurons. Nat Neurosci. 8, 1619-1626.




