Applications in drug development
Posted: 7 March 2005 |
The past decade has witnessed a growing interest in biomarkers, previously referred to as pharmacodynamic markers, PD markers, or pharmacologic read-outs. This increasing interest has been largely driven by evolutionary changes in drug discovery and development and in regulatory science1,2,3. One key driver has involved the increasing need to reach early go/no-go decisions about an increasing number of compounds entering early clinical development each year.
The past decade has witnessed a growing interest in biomarkers, previously referred to as pharmacodynamic markers, PD markers, or pharmacologic read-outs. This increasing interest has been largely driven by evolutionary changes in drug discovery and development and in regulatory science1,2,3. One key driver has involved the increasing need to reach early go/no-go decisions about an increasing number of compounds entering early clinical development each year.
The past decade has witnessed a growing interest in biomarkers, previously referred to as pharmacodynamic markers, PD markers, or pharmacologic read-outs. This increasing interest has been largely driven by evolutionary changes in drug discovery and development and in regulatory science1,2,3. One key driver has involved the increasing need to reach early go/no-go decisions about an increasing number of compounds entering early clinical development each year.
In addition to the growing need for robust and rapid go/no-go decision-making tools to go to late-phase clinical development, other key drivers have included the desirability to generate high-quality dose-response and exposure-response relationships to enable prediction and support for dosage regimen selection. Indeed, there is substantial regulatory basis for such uses of biomarkers, e.g. to provide better understanding of a drug’s effects and interpatient variability in response, to enable subgroup analyses of efficacy and safety data from clinical trials, to provide answers to questions related to dose and dosage regimens needed for the product label and to facilitate subsequent product life-cycle development programs1. Further examples of regulatory relevance of biomarkers include the recent issuance of a FDA Guidance for Industry on Exposure-Response Relationships4 and a FDA Draft Guidance for Industry on Pharmacogenomic Data Submissions5. Thus, in addition to their applications for internal decision-making, it is now well established that biomarker data can significantly enhance the overall quality and scientific strength of regulatory dossiers.
Types and definitions of biomarkers
There are, however, numerous issues and questions around biomarkers, their definitions and applications2,3. Some of these relate to the fact that there are many different types and uses of biomarkers, while others derive from their varying levels of predictability and utility. Fundamentally, the different types include different biological hierarchies, mechanistic levels and technological platforms (Figure 1). The biological hierarchies involve the different anatomic physiologic levels at which the biomarker measurements are made, i.e. molecular, cellular, tissue, organ and whole body levels. The mechanistic levels relate to whether the biomarkers are principally reflecting changes involving pharmacologic mechanisms of action or targets, pathophysiologic pathways or disease processes, or toxicologic mechanisms of action or processes. The technological platforms refer to the different measurement platforms utilised for biomarker measurements, ranging from pharmacogenetics/genomics assays, proteomics and metabolomics, through biochemical and immunologic assays, physiologic measurements, to bioimaging. Definitions of biomarkers first appeared in literature in the late 1990s, with the most widely adopted definition being that made by the NIH Biomarkers Definitions Working Group around 20006,7.
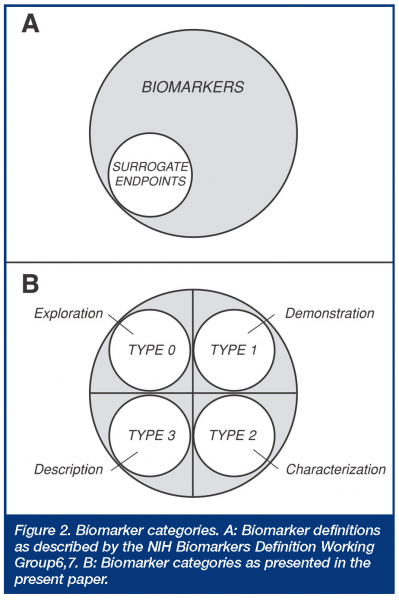
This definition was directed at a regulatory definition or statutory status of surrogate endpoints, which were defined as a subset of biomarkers. Thus, a biological marker (biomarker) was defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”, while a surrogate endpoint was defined as “a biomarker that is intended to substitute for the clinical endpoint. A surrogate endpoint is expected to predict clinical benefit (or harm or lack of benefit or harm) based on epidemiologic, therapeutic, pathophysiologic, or other scientific evidence.” While this widely used biomarker definition is straightforward regarding regulatory acceptance (surrogate endpoints), it has been criticised for being so broad that there is confusion as to what constitutes a biomarker3, and it clearly does not address general utility criteria necessary in modern drug development. Another classification, originating in the AIDS clinical trials literature, divides biomarkers into three types, based on whether they are markers of natural history describing disease severity (type 0), biologic activity markers (type I), or surrogate endpoints (type II)8,9. This classification thus divides the non-surrogate endpoint biomarkers into two mechanistic categories, without further definition of their utility. Still another classification, developed by the EU COST-B15 working group, divides biomarkers into seven types, depending on whether they represent phenotype/genotype (Type 0), concentration of drug or metabolite (Type 1), molecular target occupancy (Type 2), molecular target activation (Type 3), physiologic measures (Type 4), pathological measures (Type 5), or clinical scales (Type 6)3. This classification thus focuses on selected biologic and mechanistic levels, but again, does not address biomarker utility. More recently, several mechanistic biomarker categories have been outlined in the general literature dividing biomarkers into eight types, depending on whether they involve translation, disease, efficacy, staging, surrogate, toxicity, mechanism, or target biomarkers. How these latter types might be used in drug development or regulatory applications is not entirely clear, since there is obviously considerable potential overlap across these different descriptive categories.
Applications of biomarkers
Numerous different applications of biomarkers have been described during the past few decades, across the different pharmacologic classes of drugs and different types of biomarkers. Table 1 provides, for illustrative purposes, a list of some of the more widely recognised uses of biomarkers in drug development. While it is not the objective of this article to provide an extensive list of individual biomarkers used in drug development, as these are readily available in the literature2,6,9,10,11, a few examples will be used below as examples for the biomarker categories presented in this paper (note that many biomarkers can have more than one use). In addition, there are diagnostic, prognostic and theragnostic (Theragnostic: indicating specific therapy or drug treatment (based on thera, from therapeutics; gnostic, from knowing; also theragnosis and theragnostics, analogous to diagnosis and diagnostics) applications of biomarkers in clinical medicine, but further discussion of these is beyond the scope of this article.
Utility-based biomarker categories
For any utility-based definition, one is principally interested in knowing about the purpose of what’s being defined (what can it be used for?), the expectations (what can one do with the information obtained and with what certainty?) and the level of information derived (what is generally known about its predictability and behaviour?). Four different utility-based biomarker categories are described, based on an evidentiary or utility assessment, as follows:
- Type 0 – Exploration of drug response: Neither pharmacologic mechanism of action nor pathophysiologic pathway has a known biomarker.
- Type 1 – Demonstration of drug activity: Pharmacologic mechanism of action and/or pathophysiologic pathway have a previously described biomarker, but with an unclear relationship to efficacious dose range for a given indication.
- Type 2 – Characterisation of drug effect: Pharmacologic mechanism of action and/or pathophysiologic pathway have a well accepted biomarker, with a documented relationship to efficacious dose range for a given indication.
- Type 3 – Description of drug efficacy: Biomarker established as a surrogate endpoint (either based on pharmacologic mechanism of action and/or pathophysiologic pathway), or provides theragnostic (prescribing) information.
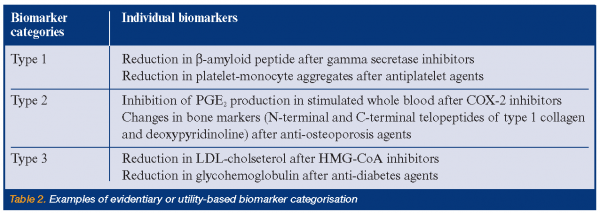
Examples of uses of this evidentiary or utility-based biomarker categorisation are shown in Table 2. Each of these four categories can have two subcategories depending on whether the biomarker is representative of a pharmacologic mechanism of action or target (subtype A) or a pathophysiologic or disease process (subtype B). What principally distinguishes Type 2 from Type 1 biomarkers is that the former category is associated with specific and documented changes, e.g. specific percent inhibition, that are indicative or suggestive of an efficacious dose range for a given indication and are suggestive of efficacy, whereas the latter does not have the same evidentiary support, although it may provide information regarding mechanism, but not necessarily efficacy. Type 1 category thus corresponds approximately to what’s called ‘‘probable valid biomarker’’ in a recent FDA’s draft guidance, whereas Type 2 corresponds to what’s called “known valid biomarker” 5; these have also been called “emerging” and “established” biomarkers, respectively. Type 0 biomarkers, on the other hand, are not associated with any a priori specific expectations of response; they are exploratory in nature, although potentially hypothesis generating. Type 3 biomarkers, however, cover all known surrogate endpoints accepted by regulatory authorities, as well as those indicating specific therapy, e.g. tumors overexpressing the HER2 protein for Herceptin® (trastuzumab). Thus, Type 3, as defined here, involves all biomarkers having critical registration and prescription implications and is thus wider than the NIH Working Group definition, which is limited to surrogate endpoints. The present categorisation expands on the biomarker definitions made by the NIH Working Group, where non-surrogate endpoint biomarkers are now divided into three categories (Figure 2).
Current trends and future directions
The main purpose of the evidentiary or utility-based biomarker categorisation presented in this paper is to provide a conceptual framework for the use of biomarkers during drug development. As was discussed earlier, there are numerous issues and questions around optimal uses of biomarkers, some of which relate to the lack of clear definitions and therefore clarity around optimal development of biomarker strategies. It is hoped that the present definitions will facilitate a more scientifically grounded discussions around the use of biomarkers in drug development. It is noteworthy that the number of biomarkers known today in each of the four categories presented varies greatly, from very few for Type 3 to possibly thousands for Type 0, particularly if one considers potential patterns or profiles involving multiple biomarkers. Also of note is that recent technological and scientific advances have very much been an enabler of rapid developments in biomarker measurements, principally in the ‘omics’ arena (see Johan Gottfries’ article in this issue of European Pharmaceutical Review), but also in bioimaging. As more information is gathered about individual biomarkers, whatever category they may be in at the present time, they may be expected to move from one category to another. Thus, there is a general expectation that many biomarkers, but by no means all, will slowly move from Type 0, through Type 1 and possibly to Type 2, with increasing knowledge and experience about their utility. Further, with respect to the number of Type 3 biomarkers, specifically surrogate endpoints, there is currently no authoritative list available of accepted surrogate endpoints. It is anticipated, however, that such a list, as well as the criteria that led to their designation, will become available in early 2005. It is also expected that the drug development paths required to validate biomarkers as surrogate endpoints will be delineated in the very near future, as part of FDA’s Critical Path initiative12. In conclusion, this paper has presented a biomarker categorisation to provide a utility-based framework for biomarker applications in drug development. With the increasing output of drug candidates from discovery, often involving novel and untested mechanisms and clinical conditions of high unmet medical need, there is an increasing need for high utility biomarkers to enable rapid evaluation of compounds emerging from drug discovery. There is also an increasing need for the development of a comprehensive database on biomarkers, with appropriate assessment and annotations relating to biopathways and disease association to hasten the progress of this important field, ideally involving collaborative efforts between academia, industry and regulatory authorities. This might best be accomplished using a consortium formation. Such an initiative is certain to lead to more efficient drug development and accelerated development of safe and effective medicines for chronic diseases. 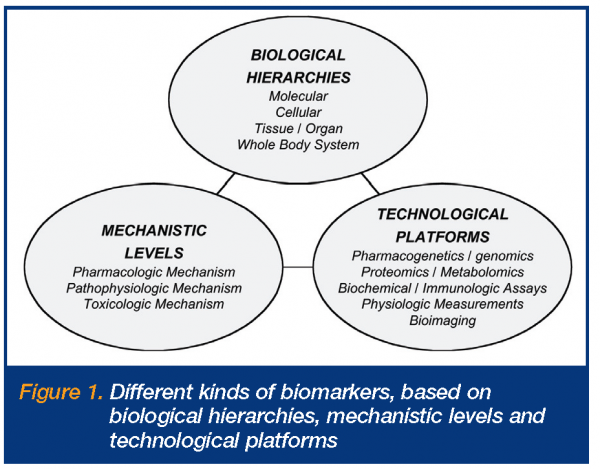





References
- Lesko LJ, Atkinson, Jr AJ. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: Criteria, validation, strategies. Annu. Rev. Pharmacol. Toxicol. 2001; 41:347-366
- Colburn WA. Biomarkers in drug discovery and development: From target identification through drug marketing. J. Clin. Pharmacol. 2003; 43:329-341
- Rolan P, Atkinson, Jr AJ, Lesko LJ. Use of biomarkers from drug discovery through clinical practice: Report of the Ninth European Federation of Pharmaceutical Sciences Conference on Optimizing Drug Development. Clin. Pharmacol. Ther. 2003; 73:284-291
- Guidance for Industry. Exposure-Response Relationships – Study Design, Data Analysis, and Regulatory Applications. April 2003. http://www.fda.gov/cber/gdlns/exposure.pdf
- Draft Guidance for Industry. Pharmacogenomic Data Submissions. November 2003. http:// www.fda.gov/cder/guidance/5900dft.pdf
- Downing GJ, editor. Biomarkers and Surrogate Endpoints: Clinical Research and Applications. New York: Excerpta Medica; 2000
- Biomarkers Definition Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001; 69:89-95
- Mildvan D, Landay A, De Gruttola V, Machado SG, Kagan J. An approach to the validation of markers for use in AIDS clinical trials. Clin. Infect. Dis. 1997; 24:764-774
- Frank R, Hargreaves R. Clinical biomarkers in drug discovery and development. Nat Rev Drug Discov. 2003; 2:566-580
- Atkinson, Jr. AJ. Physiological and laboratory markers of drug effect. In: Atkinson, Jr. AJ, Daniels CE, Dedrick RL, Grudizinskas CV, Markey SP, editors. Principles of Clinical Pharmacology. New York: Academic Press; 2001. pp. 225-234
- Trull AK, Demers LM, Holt DW, Johnston A, Tredger JM, Price, CP, editors. Biomarkers of Disease: An evidence based approach. Cambridge: Cambridge University Press; 2002
- Innovation or Stagnation. Challenge and Opportunity on the Critical Path to New Medical Products. http://www.fda.gov/oc/initiatives/criticalpath/ whitepaper.html




