Mucosal delivery – is this the next level in drug delivery?
Posted: 4 June 2017 | Flavia Laffleur - Assistant Professor at the University of Innsbruck and Researcher at the Koch Institute for Integrative Cancer Research at Massachusetts Institute of Technology | No comments yet
Following the introduction of the concept of mucoadhesion in the 1980s, polymeric excipients became attractive for intense research purposes. Mucosal delivery as a non-invasive delivery pathway raised the bar.

Theories of mucoadhesion
Mucoadhesion is defined as the process of binding a material to the mucosal layer of the body.5
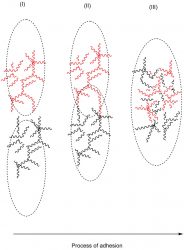

Figure 1: Mucoadhesion
concept
In Figure 1, the mucoadhesive process is displayed, showing the interpenetration of polymeric chains with the glycoprotein of the mucosal layer. This process is described by several theories.5 Starting with the oldest; wetting theory describes an embedding process in which adhesive excipients are able to penetrate the surface and establish several adhesive anchors. Within this embedding process, contact angle and the thermodynamic work of adhesion play an important part in the adhesiveness.
The second theory, namely the diffusion theory, is focussed on the interpenetration of polymeric chains of the adhesive material into the glycoprotein mucin chains. During this interpenetration, semi-permanent bonds occur while reaching sufficient depth within the matrix. The driving force for equilibrium of deep mucus penetration is a concentration gradient. By achieving an intimate contact, substrates and chains shift along their respective concentration gradients into the opposite phases. The properties of a deep diffusion are dependent on the diffusion coefficient of both phases, as well as on the molecular weight of the polymer, and decrease with increasing cross-linking density.6
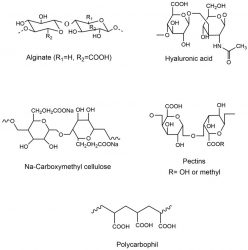

Figure 2: Anionic
polymeric excipient
Furthermore, mucoadhesion is discussed in the context of electrostatic theory, which involves a transfer of electrons along adhesive and adhering surfaces. This transfer leads to an electrical double layer maintaining a series of attractive forces for the contact between the two layers.
So-called fracture theory describes the force required for the separation of two surfaces after adhesion. This force can be determined using a tensile apparatus, where the force required to break the adhesive bond is determined.
The last, adsorptive theory, suggests the adhesion between two surfaces is adsorption after an initial contact. This theory gives the explanation of adherence due to one or more secondary forces, such as van der Waal’s forces, hydrogen bonding, and hydrophobic bonding.7
Polymers for mucosal delivery
Mucoadhesive polymers can be distinguished by their origin, whether they are naturally occurring or synthetic.8 Moreover, these polymers can be classified based on their mode of operation, such as whether they have a buccal or ocular target. They can also be categorised according to chemical structure, such as cellulose or polyacrylates, as well as their anionic,9 non-ionic or cationic behaviour. Nevertheless, mucoadhesive polymers can be divided in terms of binding mechanism to the mucosa.
Non-covalent binding polymers
The mechanism of adhesion depends on the surface charge of polymers. Anionic polymeric excipients, such as polyacrylic acid, hyaluronic acid, alginate and pectin, exhibit adhesive properties due to the carboxylic groups on their polymeric backbones (-COOH).9 Hydrogen bond formation results between the carboxylic moieties and the oligosaccharide side chains on mucus proteins.
In Figure 2 promising and well established anionic polymeric excipients are shown. The most promising polymers in mucoadhesion are reported to be polyacrylates and carboxymethyl cellulose, displaying increased buffer capacity as well as high charge density.10 One of the disadvantages of anionic polymeric excipients is their incompatibility with multivalent cations such as Mg2+ and Ca2+. Cationic polymers, on the other hand, interact with mucosa due to ionic interactions between the polymers and anionic substructures such as sialic acid groups of the mucus gel layer. Among cationic polymers, chitosan is the most investigated and well established polymer in mucoadhesion literature.11 Moreover, non-ionic polymers show the ability to form hydrogen bonds and to exhibit entanglement of polymer chains leading to mucoadhesiveness.
Covalent binding polymers
The second generation of polymers, which is able to build covalent bonds with the mucus gel layer, has been introduced to the research field. The
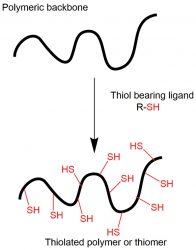

Figure 3: Thiolated
polymer- concept
of synthesis
concept of thiolated polymers, bearing thiol groups on their polymeric backbone, was introduced in the late 1990s.12 In the following years of intense research, thiomers were found to be mucoadhesive by combining the non-covalent ability to bind to mucus, as well as establishing covalent bonds in the form of disulfide bonds. This disulfide formation occurs between the immobilised sulfhydryl groups on these polymers and the thiol group in the cysteine-rich mucus glycoproteins.13 Besides their mucoadhesive properties, these polymeric excipients exhibit convincing permeation enhancing, in-situ gelling, and efflux pump inhibiting properties, focussing academic and industrial research worldwide.14 These thiomers exhibit their mucoadhesive strength between mucus and themselves due to thiol/disulfide exchange reactions, and/or a simple oxidation process. The concept of synthesis of thiolated polymers is shown in Figure 3.
Not long ago, the following class of mucoadhesive polymeric structure raised the bar. These polymers are reported to exhibit more pronounced mucoadhesive properties, as well as higher stability towards oxidation compared to corresponding polymers .15 In Figure 4, the concept for preactivation or fully S-protected thiolated polymers is displayed. This concept developed from the covalent chromatography. Peptides and proteins containing at least one cysteine subunit in their amino acid sequence are very fast and quantitatively attached to thiol-bearing resins when they are preactivated via pyridyl substructures.
In the concept of preactivation, a disulfide exchange between -SH group of thiolated polymer and nicotinamide group takes place.1 By this reaction, thiol groups are fully protected and less susceptible to oxidation, giving these preactivated derivates augmented mucoadhesive power, as well as being more reactive and stable in solutions and gels over a broad pH range.15
Concluding remarks and future perspective
Mucosal delivery systems for macromolecular therapeutics and antigens still face considerable challenges from bench to market, despite having been
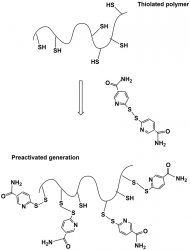

Figure 4: Preactivated or fully protected thiolated polymers – the next generation of polymers
widely investigated and shown promising research results. For this reason there is an urgent need for further understanding of the mucosal delivery mechanisms leading to enhanced drug delivery systems, which are tailor-made for specific applications. Moreover, the tailor-made component brings mucosal delivery to the next level. Polymeric excipients synthesised for specific mucosae and desired release profile will be the outcome in the not-too-distant future.
In concluding remarks, smarter mucosal devices that embrace mucoadhesion, mucus penetration, highest loading efficiency, and long-lasting strength will be highly beneficial. They will enrich the potential for sustained delivery of drugs and genes for the treatment of mucosal diseases – including those affecting the eye, respiratory tract, gastrointestinal tract and female reproductive tract, due to the inclusion of high-throughput processes, predictive mathematical models, and in-vitro and in-vivo assays. Thus, the development of mucosal dosage forms for the delivery of macromolecular drugs is considered a key challenge of significant scientific value, and simultaneously a business breakthrough for the biopharmaceutical industry. We can expect to see mucosal products in the market in upcoming years. These dosage forms must be stable in biological media, biocompatible as well as biodegradable, non‐toxic and non‐immunogenic, have mucoadhesive properties, pass mucosal barriers while shielding payload and delivering it in a controlled manner, thus increasing its bioavailability and efficacy.
Biography
Dr Flavia Laffleur is a researcher and assistant professor at the Department of Pharmaceutical Technology in Innsbruck, Austria. Dr Laffleur has published over 57 pieces of research as review articles and presented her work within several oral presentations on international conferences. From 2010 until 2013 she completed her doctoral thesis focused on smart drug delivery systems. Since 2013, she has been a senior researcher at the Department of Pharmaceutical Technology in Innsbruck. Since 2017, Dr Laffleur has been a researcher at the MIT, in Boston, Massachusetts. She has received several awards, including the Lesmüller-Stiftung award and the Galenus Foundation Technology Award. Currently, Dr. Laffleur’s research focuses on mucosal drug delivery, as well as smart delivery systems to overcome biological barriers.
References
- Laffleur F. Thiomers, mucoadhesion and oral delivery of biomacromolecules. Curr. Drug ther. [Internet]. 2014;9:56-62.
- Kharenko EA, Larionova NI, Demina NB. Mucoadhesive drug delivery systems (Review). Pharm. Chem. J. 2009. p200-8.
- Boddupalli B, Mohammed Z, Nath R, Banji D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010. p381.
- Caramella CM, Rossi S, Ferrari F, Bonferoni MC, Sandri G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015. p39–52.
- Carvalho FC, Bruschi ML, Evangelista RC, Gremião MPD. Mucoadhesive drug delivery systems. Brazilian J. Pharm. Sci. 2010. p1-17.
- Shaikh R, Raj Singh TR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. [Internet]. 2011;3:89-100.
- Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005. p1666-91.
- Laffleur F. Mucoadhesive polymers for buccal drug delivery. Drug Dev. Ind. Pharm. [Internet]. 2014;40:591-8.
- Laffleur F, Wagner J, Barthelmes J. A potential tailor-made hyaluronic acid buccal delivery system comprising rotigotine for Parkinson’s disease? Med. Chem. (Los. Angeles). 2015;7:87-90.
- Takeuchi H, Thongborisute J, Matsui Y, Sugihara H, Yamamoto H, Kawashima Y. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Adv. Drug Deliv. Rev. 2005. p1583–94.
- Khutoryanskiy VV. Mucoadhesive Materials and Drug Delivery Systems. Mucoadhesive Mater. Drug Deliv. Syst. 2014.
- Bernkop-Schnürch A. Mucoadhesive systems in oral drug delivery. Drug Discov. Today Technol. 2005;2:83-7.
- Laffleur F, Bernkop-Schnürch A. Strategies for improving mucosal drug delivery. Nanomedicine (Lond). [Internet]. 2013;8:2061-75.
- Bonengel S, Bernkop-Schnürch A. Thiomers – From bench to market. J. Control. Release. 2014;195:120–9.
- Ijaz M, Bernkop-schnu A. Preactivated thiomers: their role in drug delivery. Int. J. Pharm. [Internet]. 2015;503:1-13.




