Quality Assurance / Pharmaceutical Quality Systems in manufacturing medicinal products
Posted: 12 September 2018 | Anastasia Petropoulu | 1 comment
Quality Assurance (QA) covers all aspects that could have an impact on the quality of prescribed pharmaceutical products. This article focuses on some of the Pharmaceutical Quality Systems in relation to QA of manufactured medicines.


Quality Assurance (QA) is a wide concept and covers all aspects that could have an impact on the quality of prescribed pharmaceutical products. The objectives of QA are: to ensure that the prescribed medicine competently provides the desired effect to the person taking it; to protect patients from accidentally being administered an incorrect or contaminated medication; and to ensure medicines comply with the regulation.
Pharmaceutical Quality Systems (PQS) consist of eight pillars, which are designed to provide high quality finished pharmaceutical products, with QA and PQS working together in synergy (Figure 1). Pharmaceutical companies strive to provide high quality products to enable them to enhance their reputation, maximise profit and to provide high quality drugs to humans and animals. To meet these targets, they rely on well-designed PQS, which involve the coordination of quality through processes, with the aim of producing finished products of the highest quality.1
It is worth noting that the European Medicines Agency (EMA) defines PQS as: “The degree of excellence processed by an item” and “Meeting the requirements of specific customers’ needs”.6
The general model of controlling quality involves standards. Those include: checking the value or degree of the set standards, checking the product for conformity and feeding this back into the initial system and checking stages.2 The control of quality is an essential process and should be applied at all manufacturing stages; starting with the design, through to assembly of raw materials, in-process, post process and finally the finished products including stability testing. This explains why Quality Control is often described as being the most appropriate Total Quality Control (TQC) concept (Table 1).4,3
This article will focus on some of the Pharmaceutical Quality Systems in relation to QA of manufactured medicines. As mentioned previously, the eight pillars of PQS constitute a good foundation for discussion (Figure 1).7
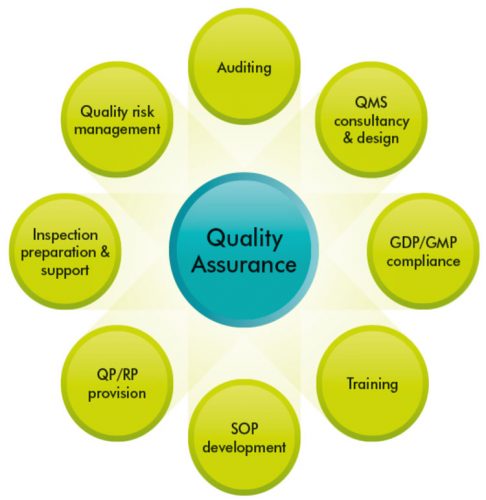

Figure 1: Eight Quality Systems contribute to the high quality of the finished pharmaceutical product
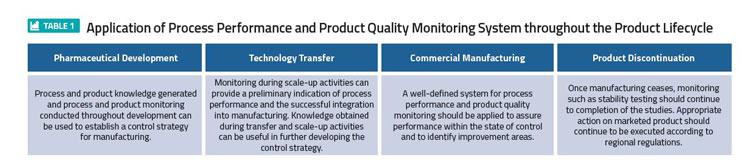
The application of a process performance and product quality monitoring system throughout the product lifecycle is shown in Table 1. This illustrates the most effective monitoring system that provides assurance of the continued capability of processes and controls to produce a product of desired quality and to identify areas for continual improvement, according to PQS Q10.5
Nevertheless, it is not possible to mention high quality finished pharmaceuticals without mentioning Good Manufacturing Practice (GMP) and Validation.5 It is well known that all manufacturing stages need quality assurance actions to ensure successful results; but how can they be achieved, and which is the most important action during all the manufacturing stages?

The answers can be found by applying GMP in each step of the manufacturing process.3 GMP is part of Quality Management that ensures products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the marketing authorisation or product specification.3 Furthermore, it ensures the manufactured products meet the end-user’s needs in terms of safety, quality and efficacy. GMP involves monitoring of processes, equipment, personnel and the environment in pharmaceutical companies.4
GMP is essential in all cases from initial drug trials to commercial launch. To obtain the best product, a manufacturer needs a system in place to ensure regular formulation, processing and composition.4 Without regulation of a manufacturing process, the consequences cause confusion that might escape notice in the first instance but at some later point will invalidate the safety of the product. This means someone will get harmed or it will cost the manufacturer money. However, the importance of patient safety is what drives companies to improve quality and prevent unnecessary expenditure on manufacturing.4
GMP applies to all types of pharmaceuticals. For example, a ‘standard product’ is one in which the unit operation and risk assessment of the end product suggests simple equipment ambient conditions; however, this doesn’t mean that the system can be abused. GMP should be applied, and the product manufactured, according to highly-regimented and regulated procedures.4 On the other hand, sterile medicines require different processes and equipment.4,2 These types of manufacturing processes often include biotechnology derivatives; where the consistency and potency of bio-preparation, that needs validation and constant monitoring, is often highly variable but may also be associated with issues such as purity.2 Sterile manufacture tends to be more vigorous in terms of equipment and specialised clean rooms. These specialised conditions and the nature of the drug itself often require additional staff training and a stronger reliance on the Qualified Person (QP) to sign-off.4
Figure 2 shows how Quality by Design embraces an integrated science and risk-based approach with continuous improvement for the entire product lifecycle.8 Process validation is needed to underpin confidence in the compatibility and coherence of each individual stage in a process of manufacture of pharmaceuticals.4
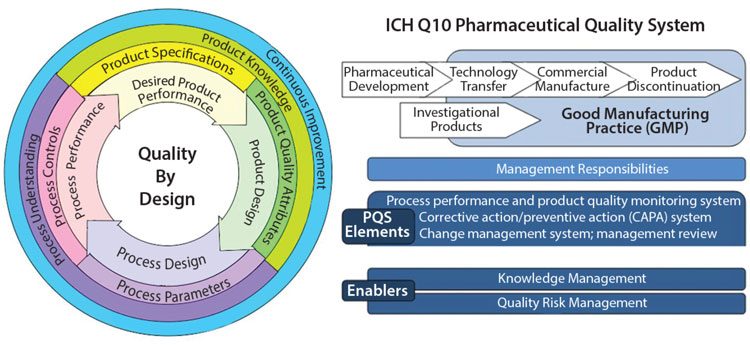

Figure 2: Challenges in Implementing Quality by Design: An Industry Perspective
This represents the biggest part of the validation process in pharmaceutical products. However, cleaning and analytical validation are equally as important in manufacturing validation as in-process, or on-process, control. The aim is to ensure end-product suitability by fragmenting the process into modules with an appropriate consideration of risk and non-compliance to established standards.4 As such, the essential considerations of any validation of manufacturing should include:
- The importance of following and establishing an environment of GMP
- The site / building / equipment limitations, aspects associated with packaging / storage / handling of the product
- The complexity of routine production, provision of a suitable audit trail in terms of detailed reports and records.3,4
Additional aspects of higher-end quality in a manufacturing validation include: the probability for consistency of manufacturing and the consequences of inconsistency. Another parameter in the validation process is the use of a pilot trial to identify the point of ‘weakness’ in a particular stage of a manufacturing process where particular attention is required.4 Pharmaceutical companies should have a system for implementing corrective and preventive actions arising from the investigation of complaints, product rejections, non-conformances, recalls, deviations, audits, regulatory inspections and findings, trends from process performance and product quality monitoring. A structured approach to the investigation process should be used, with the objective of determining the root cause.3,4
A Quality Risk Management system (Figure 3) involves monitoring and assessing the system’s or procedure’s effectiveness. This mainly involves investigating deviations that have occurred during any step of the manufacturing process, or identifying other factors such as damaged or faulty raw materials, devices or equipment.
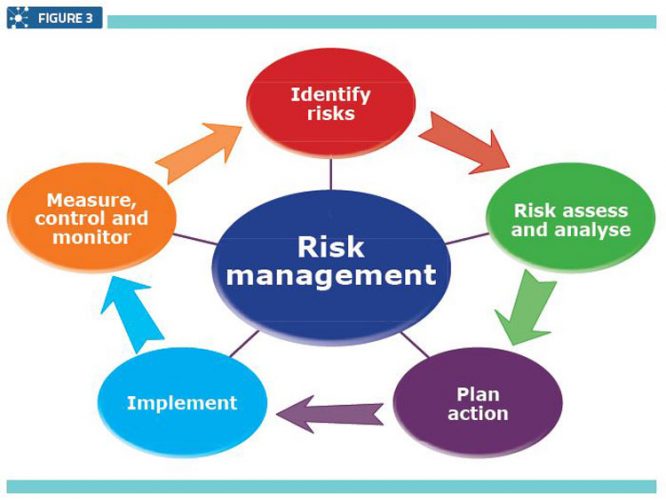

Quality risk management
The root cause analysis is identified and documented and finally an evaluation is undertaken to confirm quality objectives were achieved and the quality of the product was not affected.2 In other words, this system was built to ensure the quality of products by solving various issues or identifying risks and preventing the same happening again.
Risk management principles are used in many areas of business, including pharmaceuticals. The manufacturing and use of medicinal products, including its components, involves some degree of risk, whereas the risk to its quality is just one part of the overall risk.4 A robust quality risk management programme can ensure the high quality of pharmaceuticals by providing a proactive means of identifying and controlling potential quality issues during development and manufacturing. Effective quality risk management can provide regulators with greater assurance of a company’s ability to deal with possible risks and can positively affect the level of direct regulatory oversight.4,6
Efficient quality management results from the correct interfacing of quality control, quality assurance and quality improvement initiatives. It is achieved through acting on feedback from the people involved in the product supply chain. A quality cycle is a group of experts who meet with the aim of improving the quality of manufacturing processes, the environment, health and safety etc. Effective communication between the investors in the group can result in an improvement over and above those routine improvements.4,6
Summary
In pharmaceutical manufacturing, QA is the parameter used to ensure prescribed medicine effectively produces the desired effect on the person taking it. The PQS, part of QA system, was designed to help manufacturers achieve the target for high quality finished pharmaceutical products; leading to the required level of drug regulations and providing efficacy and safety for patients.4 The parameters for approaching these targets include:
- The pharmaceutical product is designed to meet the need and performance requirements
- The process is designed to consistently meet product critical quality attributes8
- Processes, equipment, personnel and deviations are identified and controlled in an appropriate manner
- The whole manufacturing process is constantly monitored and updated to enable consistency in quality over time.
The application of Pharmaceutical Quality Systems in pharmaceutical products can extend to pharmaceutical development, which should facilitate innovation and continual improvement of prescribed medication.2,6 It is the tool with which to achieve product realisation by designing, planning, implementing, maintaining and continuously improving a system, to allow the consistent delivery of pharmaceuticals with appropriate quality attributes.4,6
REFRENCES
- Article: Viper. 2015. Quality In Pharmaceutical Industry Management, UK essays
- Book: Beaney AM. 2006. Quality Assurance of Aseptic Preparation Services, fourth edition, published by pharmaceutical press
- Book: MHRA. 2017. Rules and Guidance for Pharmaceutical Manufacturers and Distributors, London Pharmaceutical Press, Chapter 2 EU Guidance on Good Manufacturing Practice
- Book: Sarker DK. 2008. Quality Systems and Control for Pharmaceuticals, School of Pharmacy and Biomedical Sciences University of Brighton, UK
- Article: ICH Harmonised Tripartite Guideline. 2008. Pharmaceutical Quality Systems, Q10, step 4 version
- Article: EUROPEAN MEDICINES AGENCY, science medicines health. 2015. EMA/CHMP/ICH/24235/2006, ICH guideline Q9 on Quality risk management, step 5
- Image: Morley L, CEO, EUSA, Pharma. 2016. Total Quality Service Management, Regulis, UK
- Image: Torres L. 2015. Challenges in Implementing Quality By Design: An Industry Perspective, BioProcess International
- Image: Quality Risk Manager for Manufacturing Systems; a Contamination Control Perspective, T. PDA Technical Report No 54-5 (TR 54-5)
BIOGRAPHY
Anastasia Petropoulu is a Radiopharmacy Technician / Clinical Scientist in the Radiopharmacy Department of University Hospital Bristol NHS Foundation Trust, Anastasia obtained a certificate in Health and Science followed by a BSc Hons degree in Pharmaceutical Science at the University of the West of England. She has gained experience in Quality Assurance / Quality Systems (QA / QS) by completing work in both pharmacy and the food industry. Anastasia has worked in Greece in the food industry as a Quality Assurance technician and in the UK pharmaceutical industry at Norbrook Laboratories Ltd in Northern Ireland and gained experience in testing raw materials as a Quality Control Analyst. She has also worked at NHSBT Bristol and the University Hospital Bristol NHS Foundation Trust, where she assisted in the production of parenteral nutrition and cytotoxic medicines. She currently works in Radiopharmacy as a Radiopharmacy Technician / Clinical Scientist where she applies PQS in the manufacturing process of radiopharmaceuticals.
The rest of this content is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- bi-monthly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here










Very well defined about the role of quality and most of the companies should remember that quality should be built inside the system.
Thanks for such a great post Anastasia Petropoulu.