Aseptic containment strategies to meet challenges of processing new highly toxic and biologically hazardous sterile medicinal products and therapies
Posted: 7 March 2019 | Dr Holger Kranenburg - containment lead at Franz Ziel GmbH, James L. Drinkwater - Head of GMP Compliance at F Ziel GmbH | No comments yet
Active pharmaceutical ingredients (APIs) have been established in the pharmaceutical industry over the course of many years. Containment technologies – ie, those strategies developed to contain the APIs – and qualification practices based on API powder containment have primarily been based on health and safety issues related to operator exposure to APIs via inhalation and ingestion of micrograms of powder.


Active pharmaceutical ingredients (APIs) in the pharmaceutical industry have been established over the course of many years. Containment technologies – ie, those strategies developed to contain the APIs – and qualification practices based on API powder containment have primarily been based on health and safety issues related to operator exposure to APIs via inhalation and ingestion of micrograms of powder.
Today, with many new medicinal and therapeutic product types featuring different toxicological, pharmacological and biological profiles and their associated risks to process operators and patients, there is a need for new containment strategies that enable higher levels of containment. New containment solutions should be based on risk mitigation at Health-Based Exposure Limits (HBELs). Risks to humans should be considered beyond mere inhalation of a toxic powder, to include ingestion and skin contact with transdermal routes to adverse health effects. Contaminants from aerosol/vapours from filling liquid toxic products need further consideration and more research, to characterise the risks and enable more suitable containment and decontamination solutions to be applied.
Processing of APIs that are ingredients of sterile medicines and drug conjugates that include a toxin
Some APIs considered non-sterile ingredients are subjected to a filtration stage later in compounding or dissolution in formulation of sterile medicinal products; however, bioburden may impact the final product prior to filtration. If the final product is a sterile medicine and/or a biologic that uses a biological delivery mechanism, the API will also require bio-burden control during manufacture that meets GMP requirements in environmental control and monitoring that follows Quality Risk Management (QRM) principles. QRM can facilitate adaption of conventional GMP (if justified) relative to risk mitigation for a specific process that does not suit generic practice. Biological products and therapies are typically processed aseptically as terminal sterilisation has a negative impact on product efficacy. APIs and conjugates that combine an active ingredient – eg, toxic element – and a biological delivery mechanism – eg, antibodies – provide another challenge in aseptic process filling containment as the product is filled into the final sterile product container.
Containment also has a role in cross-contamination control providing a defined containment boundary where products can be processed without contamination from other areas in shared facilities. There is a defined zone where cleaning and decontamination can render the zone free of one product, so another can be processed as a separate batch of product in the same containment zone.
Toxic or biologically-hazardous pharmaceutical products present different operator exposure risks aside from inhalation or ingestion of powders. These are airborne contaminants with containment relating more to health-based exposure limits1 (HBELs) and a series of operator health effect requirements related to average or maximum daily intake, acceptable daily exposure (ADE), permitted daily exposure (PDE) and no observable effect levels (NOEL).
Antibody drug conjugates (ADCs) have a highly toxic nature where nanogram level of containment may be required. Such new product types have created the need for a new OEB containment level 6, where nanogram containment applies and new containment strategies are required.
Development of aseptic–containment technologies and qualification methodologies
In aseptic process filling there is a balance required between patient and operator safety. API containment is primarily about operator safety and negative pressure differentials can be applied to the primary containment zone as a containment measure. In aseptic processing, however, such an application of negative pressure differential to a primary containment zone could compromise patient safety, with induction of microbial contamination from the surrounding environment putting sterile products and patients at risk.
In aseptic processing through process operations without a terminal sterilisation step to eradicate intrinsic microbial contamination as a result of processing, there can be risk to patients, specifically those with compromised immune systems. By microbial proliferation over sterile product hold or storage times, bio-contamination can become clinically significant and consequently impact patients.
During aseptic processing, individual product units can become contaminated via operator interventions into barrier systems or by material transfers in and out of barriers where individual contaminated units were not detected during sterility testing for batch release, hence putting patients at risk.
The development of aseptic containment considers patient safety without compromise or added risk and applies processing in the primary product exposure zone at a positive pressure differential. Containment measures are then applied to mitigate risks of operator exposure and contamination spread to other processing areas that process different products, hence being open to cross contamination.
Aseptic containment starts with the application of a combination of primary and secondary containment barriers that operate in unison and may vary in containment design to meet specific process requirements and levels of hazard control. Control measures are required based on containment principles to provide controlled zones and containment through material entry through the secondary and primary containment zones, together with control during final product and waste exit. Within controlled barriers, containment measures of pressure differentials and protective airflow are typically applied together with safe change filter media cells used in controlled environments.
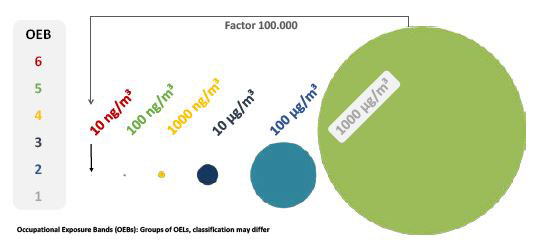

Figure 1: Occupational exposure bands (OEB). Groups of OELs, classification may differ
Primary containment barriers can be made up of closed systems – eg, vessels; containers with closed power or solution transfer connections; single-use (disposable) bags or containers with closed connections; or barrier isolator technologies designed with containment principles. Secondary containment barriers may include facility containment rooms/cleanrooms where isolator barrier systems are installed. Barrier isolators may also be used as secondary containment when used with closed or single-use systems inside the isolator that act as the primary containment.
Aseptic containment applied to the filling of sterile medicinal products is based on a contamination and containment strategy around primary and secondary containment barriers. The filling containment isolator barrier is with a Grade A/ISO5 classification for processing sterile products. The secondary containment is the surrounding Grade C cleanroom. Both primary and secondary containments should have containment attributes.
The unidirectional down-flow (UDAF) air in Grade A filling environments at 0.45m/second velocities suppresses any release of filling aerosols or freeze-dried powder from vial breakage to a lower level in the Grade A filling zone. This lower level contamination zone should be contained by the containment barrier boundary filtration and is possible to decontaminate in place. The development of new containment barrier HEPA filters that can be automatically closed for wash-in-place (WIP) and chemical decontamination, with the potential for safe change when required, is a new development designed to support containment solutions. Practice has shown it is better to ‘softly’ pre-wet the contamination zone to prevent further spread of contaminants before the ‘hard’ WIP process that removes surface contaminants to a container where the toxic product can be neutralised or denatured as a separate process.
Development of a new containment pyramid with a focus on health-based exposure risks
Aseptic process filling of toxic or biologically-active/potent products,2 including live viruses, requires a combination of contamination control GMP technical and operational control measures for patient protection and containment strategies and control measures to prevent process operators from exposure to hazardous products or ingredients.
The requirement for a contamination control strategy (CCS) as proposed in the draft (2018) revision of the EU GMP Annex 13 for manufacture of sterile medicinal products requires a side-by-side containment strategy, particularly when products are aseptically manufactured.
The balance between product, patient and process operator protection needs careful consideration, as one strategy has a direct impact on the other with the potential of adverse impact and compromise to health.4 A fundamental aspect of risk mitigation and application of containment is establishing the levels of risk to humans (process operatives and patients) so that containment solutions can then mitigate these risks.
The proposed aseptic-containment pyramid in this article is under development within the not-for-profit Pharmaceutical & Healthcare Sciences Society (PHSS)5 containment special interest group and will form part of developing guidance on aseptic containment in pharmaceutical/biopharmaceutical and therapeutic product manufacturing.
To categorise hazard levels, health-based exposure levels, and containment levels, the use of containment pyramids is a long-established practice. In the past, such pyramids have been developed around operator exposure levels (OELs). OELs at one cubic metre airflows as such consider hazards to be airborne contaminants, typically APIs, subject to inhalation.
In contrast, health-based exposure levels are set around daily intakes and exposure that may have an adverse health effect from inhalation, ingestion and skin contact with subsequent transdermal absorption. Exposure risks may be presented from aerosols from a filling process, solutions in product spills or container breakage with subsequent evaporation, and as lyophilised powders if the product is freeze dried.
When biological medicinal products or therapies are the hazard to be managed in bio-pharmaceutical and therapeutic processing, it is not good practice to consider biological safety levels (BSLs) as such levels and control measures are typically applied to uncharacterised pathogens with containment applied in research settings. BSL control measures focus on operator protection and do not follow GMP requirements for patient protection. As such, it is proposed pharmaceutical containment levels (CLs) put forward by the PHSS that provide a better platform from which to develop strategies for aseptic containment.
Development of containment solutions
There is not a single set of containment control measures that apply at a set containment level that can be defined in a containment pyramid, as such containment solutions would require development based on a case-by-case approach and driven by a containment strategy. There is an available ‘tool box’ of contamination control and containment measures that can be combined to form an aseptic–containment solution. However, when it comes to containment levels of OEB 6 there is a gap in available technologies and qualification methods to prove containment performance.
This ‘containment gap’ is being closed by new research, particularly in the area of aseptic process filling where aerosols from the filling process present a different challenge to powder containment. New containment technologies are in development to suit advanced processing requirements, together with development of new qualification methodologies and techniques, as the traditional SMEPAC containment challenge test is not sensitive enough to prove containment at OEB 6 levels and for certain types of hazardous products.
Summary
There is a need to move the thinking on from processing traditional APIs: powder containment and its associated containment technologies feature containment qualification methods that do not meet requirements for new product types and new forms of health hazard. Smaller batch, high-potency products such as biologicals or conjugates and therapies involving genes and cells (ATMPs) to combat cancer indications, provide new challenges in aseptic containment when applied to aseptic processing where immune-compromised patient risk to microbial contamination must also be managed.
The development of new containment pyramids that focus on health-based exposure and containment of different forms of health hazard, other than API powders, is important to provide a platform on which to build new containment solutions based on containment levels with technical and operational control measures that also meet EU GMP requirements for product quality, efficacy and patient safety.
Biographies




References
- European Medicines Agency; 20 November 2014: Guidance on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities.
- European Pharmaceutical Review Volume 20 Issue 3 2015: Risk-based environmental control and monitoring; James L Drinkwater, Chairman of pharmaceutical & healthcare sciences society.
- EU GMP Annex 1 (proposed revision) manufacture of sterile medicinal products.
- Presentation: PHSS Annual conference University College London, 11 September 2018: Containment and GMP: API (non-sterile) processing through Aseptic process filling of sterile products. Current status & Gap analysis. James L Drinkwater: Chairman of PHSS & Containment group leader plus F Ziel GmbH Head of GMP compliance & Aseptic processing support. Colin Newbould: Wasdell Group Director of Regulatory Affairs and QP Services. PHSS containment focus group.
- Pharmaceutical and Healthcare Sciences society: phss.co.uk
Issue
Related topics
Active Pharmaceutical Ingredient (API), Aseptic Processing, Cleanrooms, Ingredients, QA/QC









