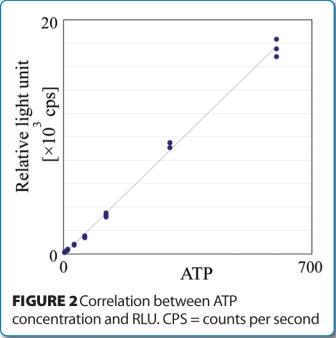
Lonza and BaroFold announce a technology evaluation agreement for PreEMT™
Lonza, a leader in biological development and BaroFold, Inc., an innovative protein technology developer, announced today a non-exclusive technology and license agreement whereby Lonza will evaluate BaroFold’s PreEMT™ high pressure refolding technology for use in biological development refolding processes.