Will Novartis provide new oral option for chronic hives?
Posted: 9 August 2023 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
If approved, Novartis’ remibrutinib has potential to be the first of a new class of chronic spontaneous urticaria (CSU) treatment in a decade.
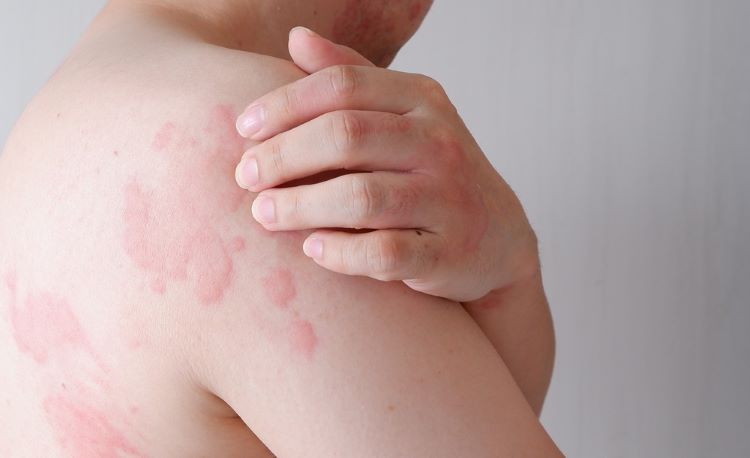

Positive top-line results from Novartis’ Phase III REMIX-1 and REMIX-2 studies showed that remibrutinib 25mg b.i.d. facilitated rapid symptom control for chronic spontaneous urticaria (CSU), also known as chronic hives, within two weeks of treatment.
The Bruton’s tyrosine kinase (BTK) inhibitor was evaluated in patients with symptoms inadequately controlled by H1-antihistamines.
H1-antihistamines are the current first-line treatment in CSU. Approximately 60 percent of patients are inadequately controlled with antihistamines alone.
While injectable biologic therapies are an effective option for CSU that is uncontrolled by these drugs, less than 20 percent of patients worldwide are treated with them, according to a 2016 British Journal of Dermatology paper and research shared by Novartis.
Potential of Novartis’ remibrutinib
In Phase II studies, remibrutinib demonstrated fast onset of action and sustained efficacy in patients with moderate to severe chronic spontaneous urticaria (CSU).
As a highly selective BTK inhibitor, remibrutinib “has the potential to be a first-in-class, oral treatment for people living with CSU whose symptoms are refractory despite use of antihistamines,” stated Dr Shreeram Aradhye, President of Global Drug Development and Chief Medical Officer at Novartis.
If approved, remibrutinib could become an effective oral option to complement Xolair® (omalizumab), the first and only injectable biologic indicated for CSU. In post-hoc analyses from pivotal Phase III registration studies, Xolair demonstrated a 78 percent improvement in quality of life for patients.
The REMIX data for Novartis’ novel BTK inhibitor will be presented at an upcoming medical meeting. The pharmaceutical company announced that it plans to submit to global health authorities in 2024.
There were 470 participants in the REMIX-1 study. In REMIX-2, the clinical trial enrolled 455 participants with CSU.
Treating other immune-mediated conditions
While CSU is estimated to affect 40 million people worldwide, Novartis also shared that remibrutinib continues to show a favourable safety profile for several immune-mediated conditions including multiple sclerosis, hidradenitis suppurativa, as well as peanut allergy.
Related topics
Big Pharma, Biologics, Biopharmaceuticals, Clinical Development, Clinical Trials, Data Analysis, Drug Development, Drug Safety, Research & Development (R&D), Therapeutics









