Small molecule drug shows promise in rare disease
Posted: 15 September 2023 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
In a Phase II trial, the small-molecule drug CBL-514 demonstrated significant reduction in lipoma size and pain improvement in Dercum’s disease, a rare disorder.
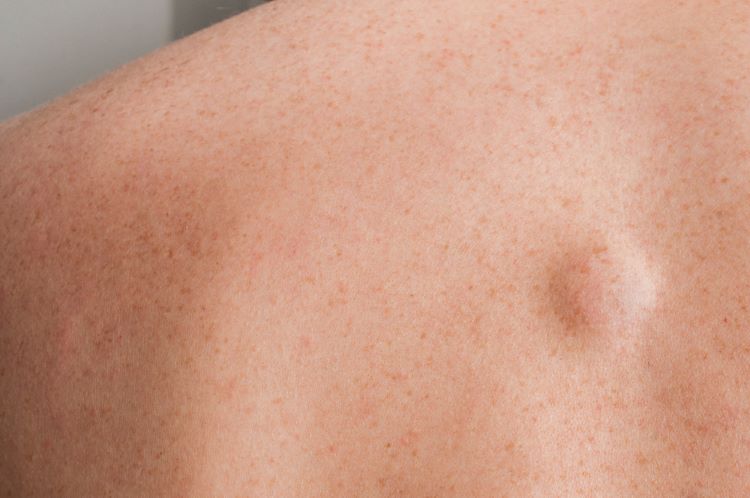

The CBL-0201DD Phase II study evaluating the first-in-class small-molecule drug CBL-514 for Dercum’s disease, a rare disorder, has demonstrated significant efficacy in reducing lipoma size and complete clearance.
Dercum’s disease is characterised by painful lipomas developing primarily on the trunk in the body. Currently there are no approved or effective medicine, so the data from the Phase II study shows potential of a treatment for this indication.
Results from the CBL-514 Phase II study
CBL-514 is the first and only product to show clinically meaningful and statistically significant in painful lipomas complete clearance or dimensions reduction of more than 50 percent”
“The topline results of CBL-0201DD [Dercum’s disease] study demonstrated that CBL-514 is the first and only product to show clinically meaningful and statistically significant in painful lipomas complete clearance or dimensions reduction of more than 50 percent, while also demonstrating significant pain improvement by 4.7 points,” stated Vivian Ling, CEO of Caliway Biopharmaceuticals, the company that developed the drug.
The study for Dercum’s disease evaluated the efficacy and safety of CBL-514 injections in twelve participants. As a primary endpoint, painful lipomas dimension in the high-dose group was reduced by 51.3 percent and 54.7 percent at 4 and 8 weeks after CBL-514 treatments.
Painful lipomas dimension in the low-dose group was reduced by 40.0 percent and 34.7 percent at 4 and 8 weeks after CBL-514 treatments.
For the secondary endpoints, 90.3 percent of painful lipomas in the high-dose group showed dimensions reduction after CBL-514 treatments when compared to baseline. In the Dercum’s disease patients who received the high-dose, 64.5 percent of painful lipomas demonstrated complete clearance or dimensions reduction of more than 50 percent after CBL-514 treatments compared to baseline.
Additionally, 38.7 percent of painful lipomas in the high-dose group showed complete clearance after CBL-514 treatments compared to baseline.
Promisingly, Caliway also shared that according to the Global Dercum’s Disease Market Research Report, the global Dercum’s disease treatment market size in 2021 was $11.3 billion. With a compound annual growth rate (CAGR) of 6.76 percent, the global market of Dercum’s disease treatment in 2030 is predicted to grow to $19.95 billion.
Future clinical progression for the first-in-class small-molecule drug
Detailed efficacy and safety topline results from the Dercum’s disease CBL-0201DD study will be published in the Journal of Rare Diseases. Based on the research findings, Caliway is currently planning for CBL-0202DD Phase IIb study IND application to further investigate CBL-514.
Related topics
Biopharmaceuticals, Clinical Development, Clinical Trials, Drug Development, Drug Safety, Research & Development (R&D), Therapeutics









