CHMP recommends EU approval of Roche’s subcutaneous Herceptin for HER2 positive breast cancer
Posted: 28 June 2013 | | No comments yet
“Over the last 14 years, Herceptin has revolutionised the treatment of HER2-positive breast cancer…”
Roche (SIX: RO, ROG; OTCQX: RHHBY) is pleased to announce that the European Union’s Committee for Medicinal Products for Human Use (CHMP) has today recommended EU approval of a subcutaneous formulation of Herceptin® (trastuzumab) for the treatment of patients with HER2-positive breast cancer. Since Herceptin’s first approval in 1998 this targeted medicine has been used to treat more than 1.3 million patients worldwide.
At present, Herceptin is given to patients intravenously, which takes 30 to 90 minutes per dose. By contrast, the new subcutaneous formulation of Herceptin can be administered in two to five minutes by a simple injection under the skin.1
“Over the last 14 years, Herceptin has revolutionised the treatment of HER2-positive breast cancer. Today, more than 80,000 patients in Europe receive Herceptin each year,” said Hal Barron, MD, Roche’s Chief Medical Officer and Head of Global Product Development. “EU approval of this subcutaneous form of Herceptin would provide a more convenient option for patients that potentially saves time and healthcare resources.”
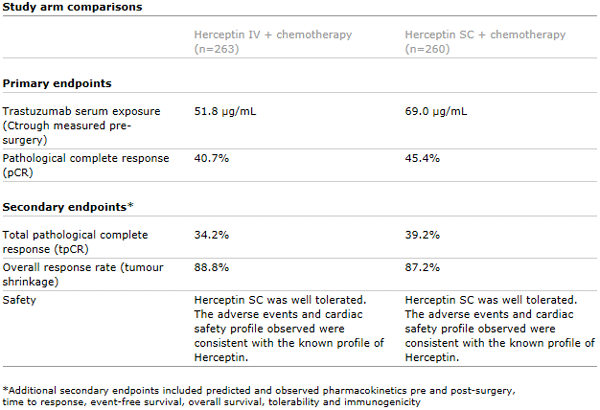
The CHMP’s positive opinion was based on data from the pivotal phase III HannaH study which showed the efficacy and safety of the subcutaneous formulation of Herceptin were comparable to treatment with Herceptin administered intravenously.1
The subcutaneous formulation of Herceptin uses technology developed by Halozyme Therapeutics, Inc. that reversibly breaks down hyaluronan, a gel-like substance that forms a barrier between cells under the skin. This enables the relatively large volume of the subcutaneous formulation of Herceptin to be rapidly dispersed over a greater area.
Roche is working with regulatory authorities around the world to ensure patients who are eligible for treatment with Herceptin have the option of choosing this more convenient therapy.
About Herceptin
Herceptin is a humanised monoclonal antibody, designed to target and block the function of HER2, a protein produced by a specific gene with cancer-causing potential when it is overexpressed. The mode of action of Herceptin activates the body’s immune system and suppresses HER2 signalling to target and destroy the tumour.
Herceptin has demonstrated unprecedented efficacy in treating both early and advanced (metastatic) HER2-positive breast cancer. Given on its own as monotherapy as well as in combination with or following standard chemotherapy, Herceptin has been shown to improve overall survival, response rates and disease-free survival while maintaining quality of life in women with HER2-positive breast cancer. Eligibility for Herceptin treatment is determined by a diagnostic test, saving time from the outset by identifying those patients who would derive greater benefit from alternative treatments.
Herceptin is marketed in the United States by Genentech, in Japan by Chugai and internationally by Roche.
The subcutaneous formulation of Herceptin is a ready-to-use liquid formulation that is administered as a 600 mg/5 ml fixed dose with a three weekly regimen. This simplifies healthcare procedure, removing the need for reconstitution or dose calculation according to individual patients’ body weights. A loading dose is not required when using subcutaneous administration.
About HannaH
Published in The Lancet Oncology in 2012, the HannaH study showed that the subcutaneous formulation of Herceptin was associated with comparable efficacy (pathological complete response, pCR) to Herceptin administered intravenously in women with HER2-positive early breast cancer and resulted in non-inferior trastuzumab serum exposure. Overall, the safety profile in both arms of the HannaH study was consistent with that expected from standard treatment with Herceptin and chemotherapy in this setting. No new safety signals were identified.

About breast cancer
Breast cancer is the most common cancer among women worldwide. Each year, about 1.4 million new cases of breast cancer are diagnosed worldwide, and over 450,000 women will die of the disease annually.2 In HER2-positive breast cancer, increased quantities of the human epidermal growth factor receptor2 (HER2) are present on the surface of the tumour cells. This is known as “HER2 positivity” and affects approximately 15-20 percent of women with breast cancer.3 HER2-positive cancer is a particularly aggressive form of breast cancer.4
References
- Gustavo Ismael, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I–III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncology, published online August 2012.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr.
- Wolff A.C et al. American Society of Clinical Oncology/ College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch Pathol Lab Med—Vol 131, January 2007.
- Slamon D et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N Engl J Med 2011; 365:1273-83.









