GSK acquisition to advance potential best-in-class GI tumour treatment
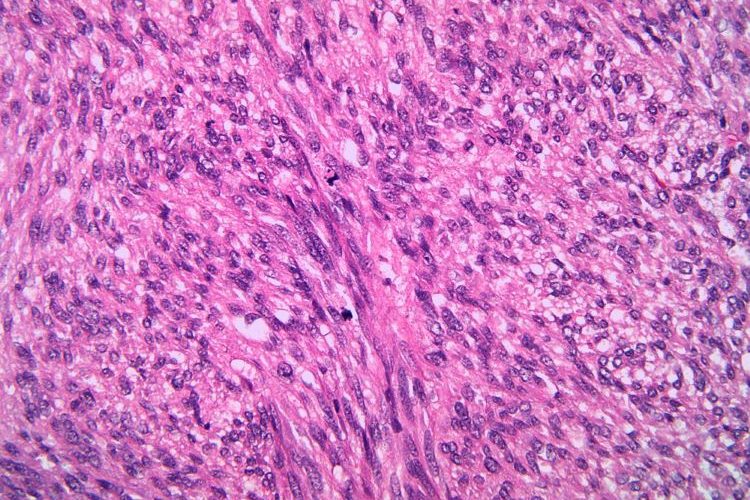
The new acquisition by GSK could enable major advancement in the standard of care for gastrointestinal stromal tumours (GIST) after nearly two decades.
GSK has agreed to acquire the biopharmaceutical firm IDRx Inc, a company focused on development of precision therapies for gastrointestinal stromal tumours (GIST).
The deal is valued up to $1.15 billion, which includes an upfront payment of $1 billion. IDRx Inc could receive a further $150 million, subject to achievement of future regulatory approvals.
IDRX-42, a highly selective small molecule tyrosine kinase inhibitor (TKI) is being developed as a first- and second-line therapy treatment for all key KIT gene mutations in gastrointestinal stromal tumours, GSK explained.
Potential of the the tyrosine kinase inhibitor in gastrointestinal stromal tumours (GIST)
Alongside demonstrated activity against all key primary and secondary KIT mutations, IDRX-42 has shown high selectivity, which could improve tolerability. These capabilities make it a potential best-in-class candidate, according to GSK.
Updated clinical findings from the ongoing Phase I/Ib StrateGIST 1 clinical trial of IDRX-42 in patients with advanced GIST, showed promising anti-tumour activity alongside a manageable safety profile. This is based on data presented at the Connective Tissue Oncology Society (CTOS) 2024 Annual Meeting.
Participants with second-line or greater gastrointestinal stromal tumours, and amongst all KIT mutation subsets, the objective response rate was 29 percent, including one complete response and 24 partial responses, the data showed.
The objective response rate was 53 percent including one complete response and seven partial responses, in patients who had one prior line of therapy.
“We are excited by the early data from IDRX-42 and its unique ability to target all clinically relevant KIT mutations present in [gastrointestinal stromal tumours (GIST)], a major gap in the current standard of care”
“We are looking forward to working with GSK to advance IDRX-42 for patients with GIST given there have been no major advances to the standard of care for almost 20 years,” stated Tim Clackson, CEO, IDRx.
“We are excited by the early data from IDRX-42 and its unique ability to target all clinically relevant KIT mutations present in GIST, a major gap in the current standard of care,” remarked Tony Wood, Chief Scientific Officer, GSK.
Alongside acquiring the biopharma company, GSK will oversee success-based milestone payments and tiered royalties for IDRX-42 owed to Merck KGaA, Darmstadt, Germany, GSK confirmed.
This transaction between GSK and IDRx Inc is subject to customary closing conditions.