Flex-FPM used to assess water contamination risk from oocyst stiffness
Posted: 8 November 2016 | | No comments yet
Oocysts from the waterborne protozoa Cryptosporidium are micrometer-sized organisms that can be highly infectious…

Oocysts from the waterborne protozoa Cryptosporidium are micrometer-sized organisms that can be highly infectious. As a consequence, many countries have mandatory monitoring procedures to detect the presence of oocysts. Upon contamination, water supplies of 100’000s of households are endangered and the water treatment plant and network need to be cleaned, which is costly and time consuming.


Not all Cryptosporidium species are infectious. The currently used detection method can identify the presence of Cryptosporidium oocysts, but has difficulty discriminating the different species and can’t detect the virulence or viability of the oocysts. Animal tests can be used for this purpose, but it takes several days to detect whether animals become infected or not.
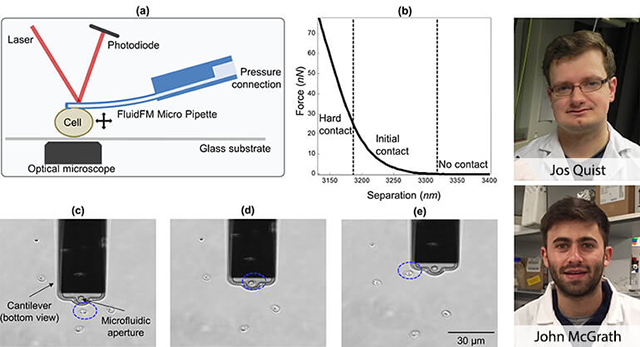
As an alternative method, researchers from Edinburgh and Twente found that the stiffness of Cryptosporidium oocysts varies between species and with viability. These findings were obtained with a Nanosurf Flex-FPM system. The Flex-FPM uses FluidFM® technology to reversibly aspire single oocysts to a hollow cantilever and measure their stiffness by squeezing them between the cantilever and a glass surface. Jos Quist from Twente University stresses the benefit of the reversible binding, allowing him to measure up to 30 cells per hour with 8 measurements per cell. This is more than 10 times faster than what can reasonably be achievable with irreversible binding of cells to a cantilever. When asked the question, why immobilization is needed at all, John McGrath from Heriot-Watt University, Edinburgh, mentioned that the cells would dislodge during the force measurements if not tightly bound to the cantilever.
The question arises, whether 30 cells per hour are sufficient for water testing in practice or not. John McGrath relates it to the low number of cells that are filtered out during 24 hours standard monitoring, in which typically thousand liters of water are filtered and concentrated. Already 1–10 oocysts from 1000 liters of water trigger a caution level, while more than 10 oocysts cause an alert. Consequently, high numbers are not available, nor required for testing. Both researchers agree that more research and instrument optimization would be required to make FluidFM® a standard tool for oocyst identification in a water treatment plant. In the meantime, John McGrath sees other applications of the Flex-FPM system, e.g. for single oocyst isolation or for the local administration of drugs to single oocysts using the hollow cantilevers. Jos Quist also points out that this type of measurement is not restricted to oocysts, but could also be used for stiffness measurements on other types of cells.
The work of John McGrath and Jos Quist has been published in PLoS ONE:
McGrath JS, Quist J, Seddon JRT, Lai SCS, Lemay SG, Bridle HL (2016) Deformability Assessment of Waterborne Protozoa Using a Microfluidic-Enabled Force Microscopy Probe. PLoS ONE 11(3): 0150438. doi:10.1371/journal.pone.0150438
Want to discover the Flex-FPM for yourself? Contact us!




