Two lots of Kogenate® recalled due to mislabelling
Posted: 24 July 2019 | European Pharmaceutical Review | No comments yet
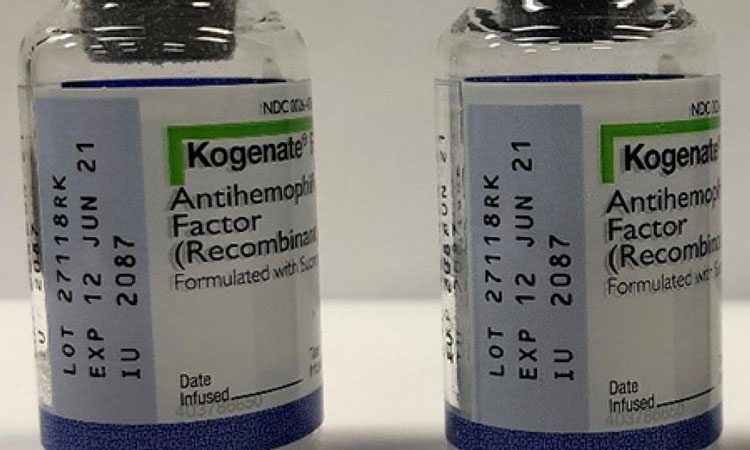
Two lots of Kogenate® FS vials actually contain the FVIII hemophilia A treatment, Jivi®.

(Source: FDA).
Bayer is voluntarily recalling 990 vials of a biological drug used to help prevent and control serious bleeding in patients with hemophilia A.
Two lots of Kogenate FS antihemophilic factor (recombinant) 2000 IU vials that were labeled as Kogenate FS actually contain the FVIII hemophilia A treatment, Jivi antihemophilic factor (recombinant) PEGylated-aucl 3000 IU.
In its statement to the US Food and Drug Administration (FDA), the company said that the US is the only country where affected products were distributed and it has said it is working closely with the administration to manage the recall and to minimise disruption to supply and inconvenience to patients.
“While the majority of the mislabelled vials in the affected lots were recovered, approximately 990 of these vials were released in the US,” said the statement. “The associated Jivi batch was expired as of August 2018. However, all stability specifications of this expired Jivi batch had continued to be met, as of April 2019. We have voluntarily recalled both lots in the interest of patient safety, and to ensure that any potentially impacted product is removed from pharmacy shelves, and that patients and their healthcare providers are alerted. Importantly, vials of Kogenate FS that are not associated with the affected lot numbers are not impacted and can continue to be used without interruption.
“There are no lots of Jivi or Kovaltry® antihemophilic factor (recombinant) product affected by this recall.”
Kogenate FS and Jivi are both medicines used to replace clotting factor (factor VIII or antihemophilic factor) that is missing in people with hemophilia A.
“Patients in possession of vials from the affected lot numbers should immediately stop using the product and contact their physician,” the statement continued. “In addition, patients should contact their pharmacy to return the affected product.”
The affected lots (a list of which can be found here) were distributed from Bayer’s distribution sites in Berkeley, CA and Shawnee, KS.









