Silica-based drug delivery could improve anticancer drugs
Posted: 16 August 2019 | Victoria Rees (European Pharmaceutical Review) | No comments yet
Researchers have developed silica spheres to contain cancer therapeutics that melt at certain temperatures and have the potential to improve drug delivery.

Jichuan Qiu, a postdoctoral fellow at Georgia Tech and Younan Xia, professor and Brock Family Chair in the Wallace H Coulter Department of Biomedical Engineering at Georgia Tech and Emory University (credit: Allison Carter).
A team has developed small temperature-sensitive silica bottles filled with medicine to be used in drug delivery to kill cancer cells. The researchers, from the Georgia Institute of Technology, US, created silica-based hollow spheres around 200nm in size with a small hole in the surface to encapsulate a wide range of payloads for release at certain temperatures.
To test them, the team placed a mixture of fatty acids, a near-infrared dye and an anticancer drug into the spheres. The acids remain solid at human body temperature but melt at a few degrees above this. When an infrared laser is absorbed by the dye, the fatty acids are quickly removed to release the therapeutic drug.
“This new method could allow infusion therapies to target specific parts of the body and potentially negating certain side effects because the medicine is released only where there’s an elevated temperature,” said Younan Xia, professor and Brock Family Chair in the Wallace H Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. “The rest of the drug remains encapsulated by the solid fatty acids inside the bottles, which are biocompatible and biodegradable.”
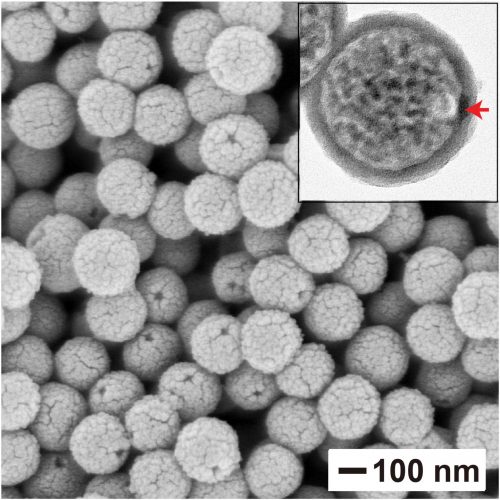

This scanning electron microscopy image shows the nanocapsules once formed after the removal of the gold nanoparticles and polystyrene beads, leaving behind an opening that can be used to fill the capsules with a payload (credit: Jichuan Qiu).
The researchers also showed that the size of the hole could be changed, allowing the nanocapsules to release their payloads at different rates.
To make the silica-based bottles, the team fabricated spheres out of polystyrene with a small gold nanoparticle embedded in its surface. The spheres were then coated with a silica-based material everywhere except in the location of the gold nanoparticle. When the gold and polystyrene are removed, only a hollow silica sphere with a small opening remains. To adjust the size of the opening, the researchers changed the size of the gold nanoparticle.
The process to load the bottles with their payload involves soaking the spheres in a solution containing the mixture, removing the trapped air, then washing away the excess material and filling with water. The resulting nanocapsules contain an even mixture of the temperature-sensitive material, the therapeutic drug and the dye.
To test the release mechanism, the researchers put the nanocapsules in water and used a near-infrared laser to heat the dye while tracking the concentration of the released therapeutic. This confirmed that without the use of the laser, the medicine remains encapsulated. After several minutes of heating, concentrations of the therapeutic rose in the water.
“This controlled release system enables us to deal with the adverse impacts associated with most chemotherapeutics by only releasing the drug at a dosage above the toxic level inside the diseased site,” said Jichuan Qiu, a postdoctoral fellow in the Xia group.
The results were published in Angewandte Chemie International Edition.
Related topics
Related organisations
Angewandte Chemie International Edition, Georgia Institute of Technology









