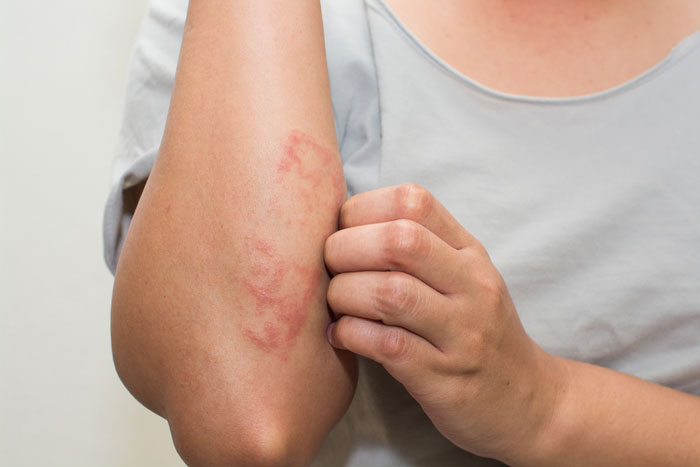
EC grants marketing authorisation of Dupixent for atopic dermatitis
Sanofi and Regeneron Pharmaceuticals, Inc. have announced that the EC…
Sanofi and Regeneron Pharmaceuticals, Inc. have announced that the EC has granted marketing authorisation for Dupixent for use in adults with moderate-to-severe atopic dermatitis...