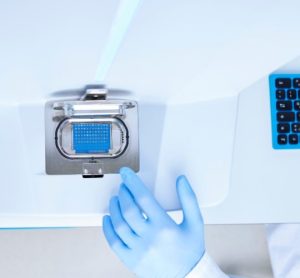
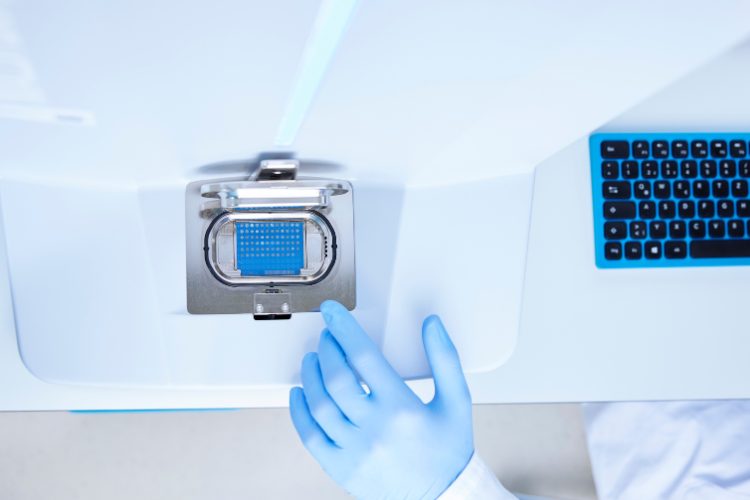
Application note: Highly efficient method development using LC-MS for automated peak tracking
Peak tracking in liquid chromatography (LC) method development can be challenging due to retention variations and co-elution. LabSolutions MD software with LCMS-2050 SQ-MS simplifies peak identification and automates method and sequence creation, enabling efficient development of reliable separation methods.