Innovating bioanalysis to advance oligonucleotide therapeutics
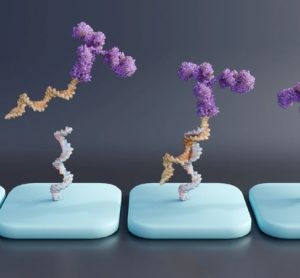
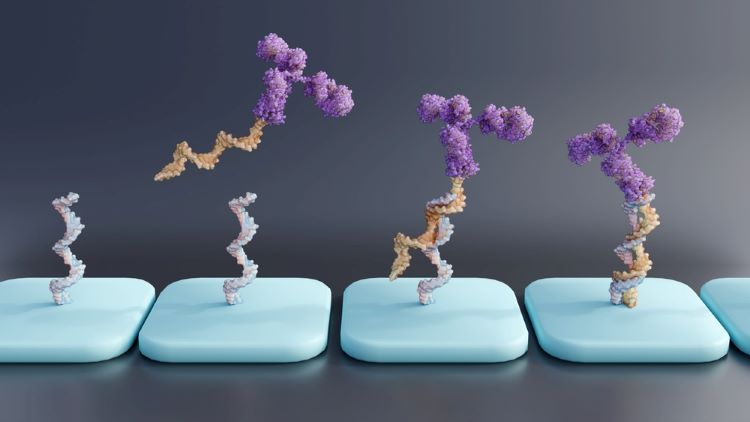
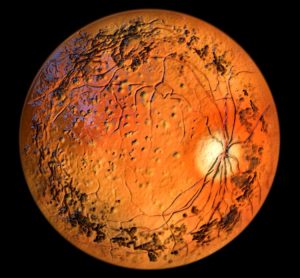
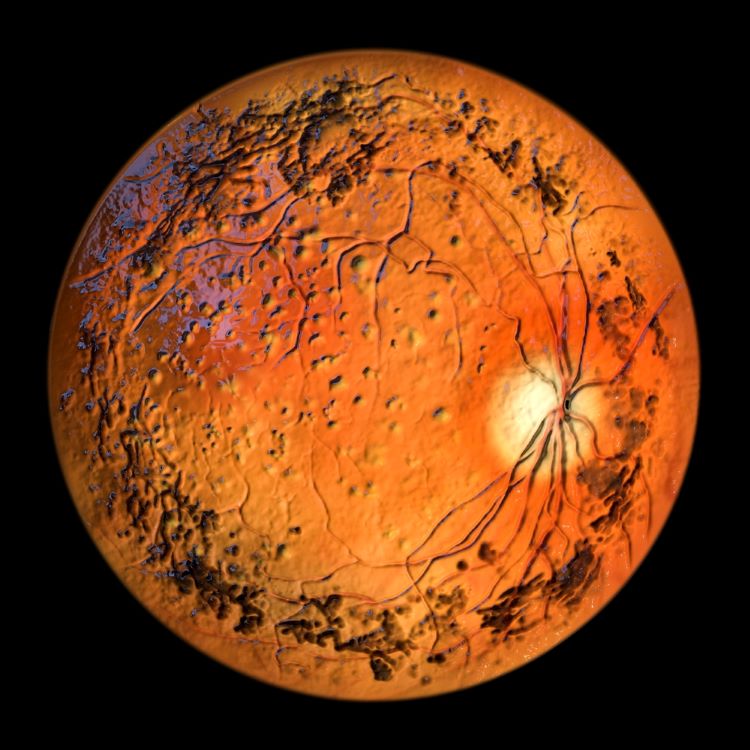
Here, Dr Jinpeng Li at WuXi AppTec reveals the analytical challenges that are hindering advancement of oligonucleotide therapeutics, medicines which have demonstrated clinical promise in the gene therapy space, and discusses promising analytical innovations.