Could a simple handle change your cleanroom contamination risk?
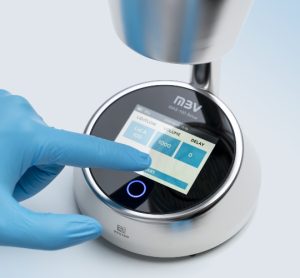
MBV AG addresses challenges in air monitoring and aseptic handling with MAS-100 Sirius®: a next‑generation active microbial air sampler created in close collaboration with pharmaceutical manufacturers and cleanroom experts.